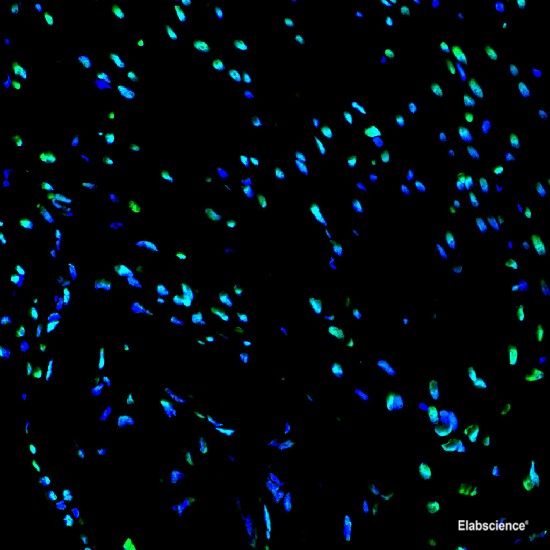
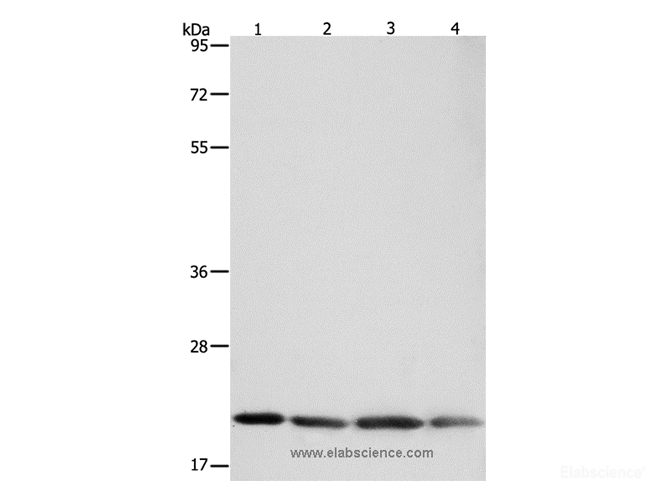
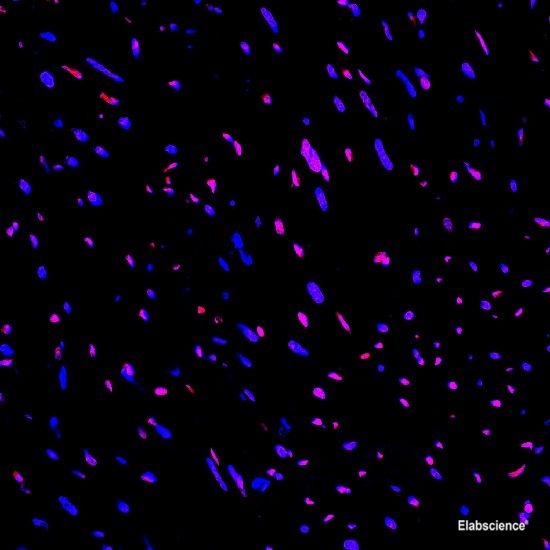
Preparation of protein samples
1.Protein extraction
1)For tissue sample
a. Take the samples, wash the tissue thoroughly with pre-cooled PBS (0.01 M, pH=7.4)(Cat# E-BC-R187) to remove the surface blood and internal debris.
b. Weigh and smash the tissue, add an appropriate ratio of RIPA Lysis Buffer (Cat# E-BC-R327)(add 10 μL PMSF and 10 μL Na3VO4 to each 1 mL RIPA Lysis) and homogenizely lyse the tissue.
It is recommended to homogenize according to the ratio of tissue weight: RIPA volume = 3:10. For example, add 1 mL RIPA Lysis Buffer to 0.3 g tissue sample, the specific volume can be adjusted according to experimental requirements.
c. Shake and lyse on the ice for 30 min after homogenization. And then sonicate the sample for 1 min (under ice water bath conditions) with 2 s’ sonication and 2 s’ intervals to make cells fully lysis and reduce the viscosity of sample.
d. Centrifuge at 12,000 rpm for 10 min at 4℃.
e. Take the supernatant and measure the protein concentration mentioned in step2.
2)For cell sample
a. Collect the cells, wash them thoroughly with pre-cooled PBS (0.01 M, pH=7.4) to remove the medium off (it is generally recommended to wash 3 times).
b. Add an appropriate ratio of RIPA Lysate Buffer (10 μL PMSF and 10 μL Na3VO4 in each 1 mL RIPA Lysis) and lyse on the ice for 30 min.
It is recommended to add 0.1 mL of RIPA Lysis Buffer to each well of a 6-well plates (the protein content in different cells may vary, and the volume of the lysate added can be appropriately adjusted).
c. Sonicate the sample for 1 min (under ice water bath conditions) with 2 s’ sonication and 2 s’ intervals to make cells fully lyse and reduce viscosity of sample.
d. Centrifuge at 12,000 rpm for 10 min at 4℃.
e. Take the supernatant and measure the protein concentration mentioned in step2.
2.Measurement of protein concentration
By the BCA method (see the Total Protein Colorimetric Assay Kit (Cat# E-BC-K318) instructions).
3.Boiling the samples
Adjust the protein concentration with PBS Buffer. Add 5 × SDS Loading Buffer (Cat# E-BC-R288) with the ratio of the protein sample: 5 × SDS Loading Buffer = 4:1 and boil the mixture for 10 min. Centrifuge at 12,000 rpm for 2 min and collect the supernatant. The denatured protein can be employed to Western Blot experiments or stored at -20℃ or -80℃.
Note: It is recommended that the total protein loading amount of test sample is about 50 μg in each well. Try to make the loading volume of each sample close to 10 μL.
Electrophoresis
1.According to the molecular weight of the target protein, prepare 0% separation gel. Add the test sample to each well, and add 5 μL of Pre-stained Protein Marker (Cat# E-BC-R273)to a reserved well in order to verify the target molecular weight and the extent of membrane transfer. Add Electrophoresis Buffer ( Cat# E-BC-R331) and start electrophoresis.
2.Electrophoresis at 80v when the samples are in stacking gel, then convert to 120v when the blue flow into the separating gel. Electrophoresis time is about 2-3 h till bromophenol blue reaches the bottom of the gel.
Transfer Membrane
1.Choose the PVDF Membrane (Cat# E-BC-R266) with a pore size of μm according to the molecular weight of the target protein. Soak the PVDF Membrane in methanol for 1 min to activate it, and then soak the PVDF Membrane in the Transmembrane Buffer (Cat# E-BC-R333), the filter paper and fiber mat must be soaked in the Transmembrane Buffer for use too.
2.Follow manufacture instructions of Transfer System for wet, semi-dry, or dry transfer.
Incubation of antibodies
1.Soak the PVDF Membrane with TBST Buffer (Cat# E-BC-R335) containing 5% Skim Milk Powder as blocking buffer and block the membrane at room temperature for .
2.According to the recommended primary antibody dilution ratio, use the TBST Buffer containing 5% Skim Milk Powder to dilute the β-Tubulin Antibody at , soak the PVDF Membrane in the primary antibody working solution, incubate overnight at 4 ℃, and gently shake.
3.Wash the PVDF Membrane with TBST Buffer for .
4.According to the recommended secondary antibody dilution ratio, use a TBST Buffer solution containing 2% Skim Milk Powder to dilute Goat Anti-Rabbit IgG (H+L) (peroxidase/HRP conjugated) (Cat# E-AB-1003) at . Incubate at room temperature for 1 h on a shaker.
5.Wash the PVDF Membrane with TBST Buffer for .
Detection
1.Mix A and B in the Excellent Chemiluminescent Substrate Detection kit (Cat# E-BC-R347) at the ratio of 1:1 as working solution.
2.Take out the PVDF Membrane from TBST Buffer and absorb the liquid with the filter paper. Pave the PVDF Membrane on the detection machine, add ECL working solution continuously on the PVDF Membrane, discharge the bubble and detect the result.
3.Adjust the contrast and the exposure time to get the best image.
Appendix
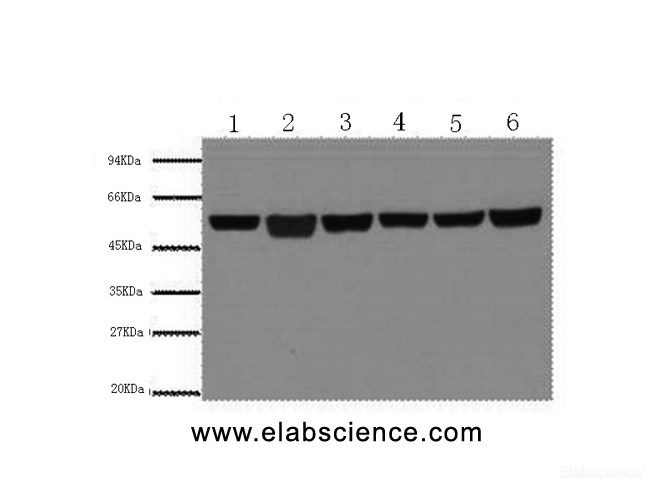
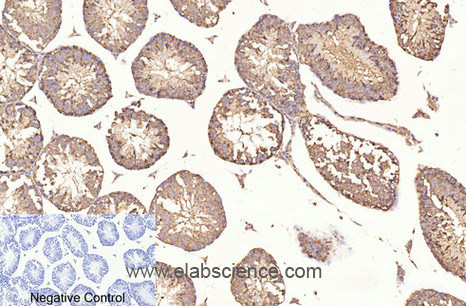
 Summary of Verified Samples
Summary of Verified Samples