The Tricarboxylic Acid (TCA) cycle is a crucial process for energy metabolism in living organisms, while cuproptosis is a newly identified form of cell death. What is the connection between them? In this post, we will uncover the connections between them.
Table of Contents
1. Background on Cuproptosis
2. Background on Key TCA Cycle Enzymes
3. TCA Cycle Dysfunction Leads to Cuproptosis
4. The Central Role of Protein Lipoylation
01 Background on Cuproptosis
A March 2022 study published in Science, titled "Copper induces cell death by targeting lipoylated TCA cycle proteins", revealed that excess copper induces cell death by directly binding to lipoylated proteins in the TCA cycle. Copper overload triggers FDX1/LIAS-mediated lipoylation, leading to oligomerization of DLAT, which is a key component of the pyruvate dehydrogenase complex. This disrupts TCA cycle function, causes proteotoxic stress, and ultimately results in cell death. This work established a direct mechanistic link between cuproptosis and mitochondrial metabolism.
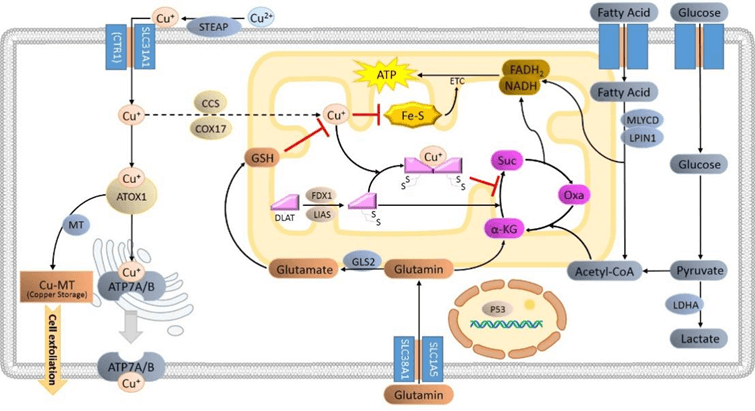
Fig. 1 Copper ion overload induces DLAT oligomerization, leading to cell death.
02 Background on Key TCA Cycle Enzymes
The TCA cycle is a central part of energy metabolism. Through its enzymatic machinery, it converts glucose, fatty acids, and amino acids into NADH and FADH2, which are then used to generate ATP via the Electron Transport Chain (ETC). Several enzymes in this cycle are lipoylated. The Tsvetkov et al. study indicated that proteins with lipoic acid groups readily bind to copper ions. In mammals, there are four primary lipoylated proteins: DBT, GCSH, DLST, and DLAT. Among them, DLAT is a vital component of the pyruvate dehydrogenase complex within the TCA cycle. Pyruvate dehydrogenase converts pyruvate into acetyl-CoA, which subsequently enters the TCA cycle.
03 TCA Cycle Dysfunction Leads to Cuproptosis
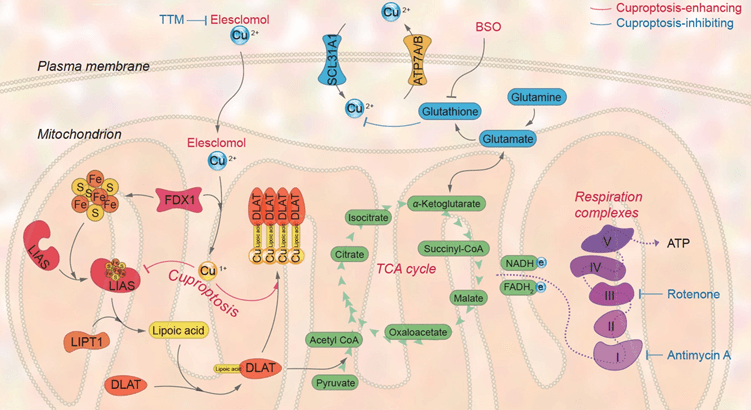
Fig. 2 Copper overload causes oligomerization of lipoylated proteins, thereby affecting the cellular TCA cycle.
04 The Central Role of Protein Lipoylation
Protein lipoylation is a specific mitochondrial modification where lipoic acid attaches to proteins. In cuproptosis, the protein FDX1 plays a dual role: it reduces copper ions and promotes the lipoylation of TCA cycle enzymes like Pyruvate Dehydrogenase (PDH) and α-Ketoglutarate Dehydrogenase (α-KDH). Studies show that cells lacking FDX1 have impaired lipoylation, leading to metabolic buildup and disrupted glucose metabolism. This explains why copper overload is so toxic: copper binds to these essential lipoylated enzymes, crippling the TCA cycle and killing the cell.
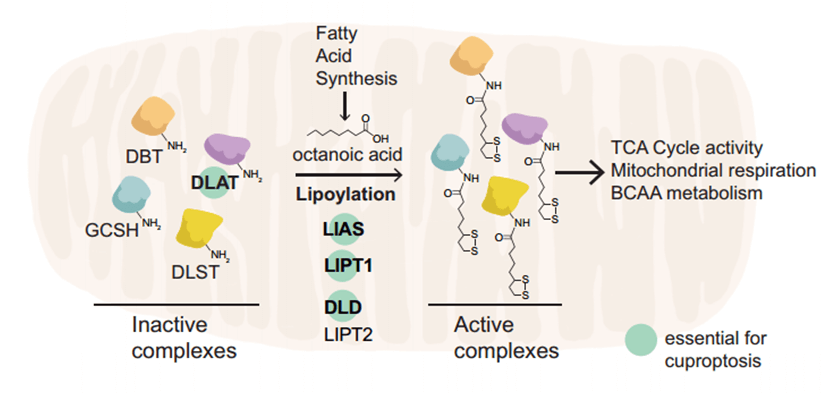
Fig. 3 Several important lipoylated proteins
In summary, the TCA cycle is a key pathway regulating cuproptosis. The primary mechanism is that copper ions bind to lipoylated enzyme components of the TCA cycle, inducing their oligomerization. This disrupts the TCA cycle pathway, leads to metabolic imbalance, triggers proteotoxic stress, and ultimately results in cell death.
For more insights into cell metabolism, stay tuned with Elabscience®!
Reference
[1] Tsvetkov, Peter, et al. "Copper induces cell death by targeting lipoylated TCA cycle proteins." Science 375.6586 (2022): 1254-1261.
[2] Duan, Wen-Jun, and Rong-Rong He. "Cuproptosis: copper-induced regulated cell death." Science China Life Sciences 65.8 (2022): 1680-1682.
[3] Tang, Daolin, Chen, and Guido Kroemer. "Cuproptosis: a copper-triggered modality of mitochondrial cell death." Cell research 32.5 (2022): 417-418.



