Introduction
A modification of the basic immunofluorescent staining and flow cytometric analysis can be used for simultaneous analysis of surface molecules;
intracellular antigens and at the single-cell level by flow cytometry. Typically, cells are fixed with formaldehyde to stabilize the cell membrane, then permeabilized with detergent or ethanol to allow antibodies against intracellular antigens, to access to stain intracellularly.
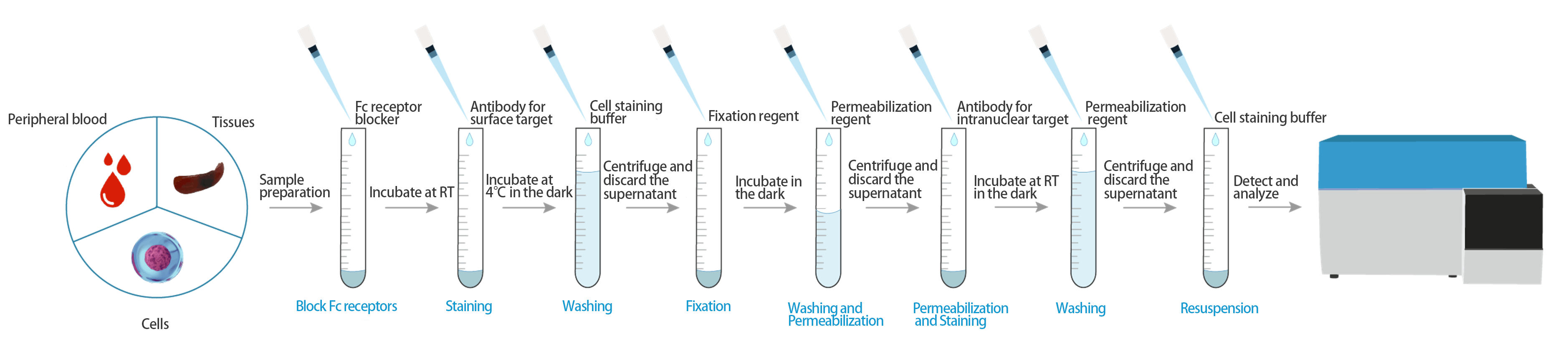
Protocol-- intranuclear proteins
1. Prepare cells
More detail you can view Sample Preparation for Flow Cytometry
(1). Collect cells, filtere through a 200-mesh sieve and collect the filtrate. Centrifuge at 300 × g for 5 min, and discard the supernatant.
(2). Add cell staining buffer [E-CK-A107] (or PBS with 1% BSA) to resuspend the sample.
2. Cell Counting
After counting the suspension with a hemocytometer or other instruments, adjust the cell concentration to about 1 × 106/mL.
3. Cell Viability Staining
(1). Add 1 μL of STYX™ Fixable Viability Dye working solution [E-CK-A171]per 1 mL of cell suspension (Note: Select an appropriate amine-reactive viability dye according to the flow cytometry panel). Mix gently by pipetting and incubate at room temperature or 4°C in the dark for 30 minutes.
(2). Centrifuge at 300 × g for 5 minutes. Discard the supernatant, resuspend the cell pellet in an appropriate volume of PBS, and centrifuge again at 300 × g for 5 minutes. Discard the supernatant, resuspend the cells in an appropriate volume of Cell Staining Buffer [E-CK-A107], and adjust the cell density to approximately 1×10⁷/mL.
4. Set Sample and Control
|
Groups
|
Tubes
|
|
Controls
|
Blank
|
|
Single staining control
|
|
|
Isotype control
|
|
|
FMO
|
|
|
Biological control
|
|
|
Sample
|
Experimental sample
|
5. Block Fc Receptor
Block Fc receptors may reduce nonspecific immunofluorescent staining.
For human cells, EasyStain™ Human Fc Receptor Blocking Solution [E-CK-A171] can be used as an FcR blocking reagent. Add 5 μL of EasyStain™ Human Fc Receptor Blocking Solution, mix well, and incubate at room temperature for 10 min.
For mouse cells, Purified Anti-Mouse CD16/CD32 Antibody specific for FcγR III/II can be used to block nonspecific staining of antibodies, and reduces the background fluorescence of negative cells to the level of unlabeled cells. Add 1 μg of Purified Anti-Mouse CD16/32 Antibody [E-AB-F0997A] and incubate at room temperature for 10 min.
For rat cells, excessive purified Ig from the same source and subtype as fluorescent antibodies or serum from the same source can be directly used for blocking, or commercial FcR blocking agents can be used.
6. Cell Surface Staining
(1). Add 5 μL corresponding antibody to each sample tube except blank.
(2). Incubate at 4°C for 30 min in the dark.
7. Fixation and Permeabilization
If the markers are those in the intracellular (e.g. IFN-γ, IL-4, IL-17), please refer to the Cells Intracellular Targets Staining for Flow Cytometry.
(1). Dilute Fixation and Permeabilization Solution[E-CK-A108] according to the manual.
(2). Add 1 mL cell staining buffer [E-CK-A107] (or PBS with 1% BSA) to each tube, centrifuge at 300 × g for 5 min, and discard the supernatant.
(3). Add 100 μL cell staining buffer [E-CK-A107] (or PBS with 1% BSA) to resuspend the sample.
(4). Add 1 mL 1× Fixation Working Solution to each tube, mix gently.
(5). Incubate at 4°C for 30 min in the dark.
(6). Centrifuge at 600 × g for 5 min, and discard the supernatant.
(7). Add 2 mL 1× Permeabilization Working Solution to each tube, mix gently.
(8). Centrifuge at 600 × g for 5 min, and discard the supernatant.
(9). Repeat (7)(8) one more time.
8. Cell Intracellular Staining
(1). Add 100 μL 1× Permeabilization Working Solution to each tube, resuspend the sample.
(2). Add 5 μL corresponding antibody to the tube required.
(3). Incubate at RT for 30 min in the dark.
(4). Add 1× Permeabilization Working Solution to each tube, resuspend the sample.
(5). Centrifuge at 600 × g for 5 min, and discard the supernatant.
9. Detection
(1). Add 200 μL cell staining buffer [E-CK-A107] (or PBS with 1% BSA) to resuspend the sample.
(2). Adjust instrument parameters, detection.



