The preparation process of human peripheral blood PBMC
1) Collect fresh human blood using heparin sodium anticoagulant tubes.
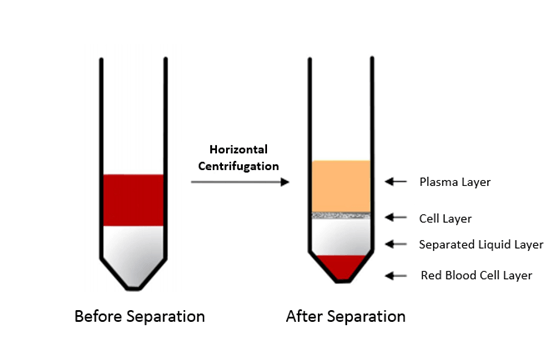
2) Add 5 mL of well-mixed Ficoll solution (1.077 g/mL) (Ficoll is taken out of the 4°C refrigerator half an hour in advance and returned to room temperature. If the temperature is too high, the separation will not be obvious, and if the temperature is too low, the density will be too large, and the separation effect will also be poor. The separation effect is best at about 20-25°C) to a 15 mL centrifuge tube, then add 5 mL of fresh blood along the tube wall slowly. The obviously stratification of blood and Ficoll liquid represents successful preparation.
3) Transfer the sample to a centrifuge and centrifuge at 500 × g for 20 min.
4) Aspirate and discard a portion of the top layer supernatant (approximately 1-2 mL). Gently pipette 1 mL of the second layer supernatant along with a small amount of the third-layer separation solution (to enhance cell yield) using a 1 mL pipette, and collect it into the same 50 mL centrifuge tube.
5) Washing: Add RPMI 1640 basal medium, mix well, centrifuge at 250 × g for 5 min, and discard the supernatant.
6) Repeat the wash step once.
7) Resuspend the cell pellet using RPMI 1640 complete medium or add the corresponding liquid as required for subsequent experiments.
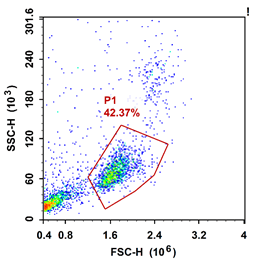
FSC/SSC diagram of human peripheral blood PBMC
Precautions:
1. The temperature of Ficoll is very important. Too high or too low temperature will affect the separation effect. The optimal temperature is 20-25ºC.
2. The blood sample should preferably be freshly anticoagulated (within 2 hours after blood collection). To maintain cell viability, freezing and refrigeration should be avoided.
3. In order to obtain the maximum amount of mononuclear cells, it is better to use a 15 mL centrifuge tube rather than 50 mL, and the amount in the centrifuge tube should not exceed one third of the centrifuge tube. Blood samples can be aliquoted and added to multiple centrifuge tubes.
4. For cell cytokine detection, directly inducing and blocking the sorted cell sample yields optimal results. Alternatively, the sorted samples can be cryopreserved and induced for detection when needed, with no significant difference in performance compared to direct induction (provided the storage time is kept within one week).
5. For intracellular factors detection, samples that cannot be detected in time after induction are recommended to be stored frozen, and the detection effect is best within 3 days. It is recommended to use 90% FBS + 10% DMSO as a freezing medium. After 24 hours of cryopreservation and retesting, the results were not significantly affected. After 3 days of cryopreservation, the expression of CD3 and IFN-γ decreased slightly. After 1 week of cryopreservation, the expression of CD3 and IFN-γ decreased significantly.
6. Picture of Ficoll after separation: