Breast cancer (BC) still represents the most frequent cancer in women and the second cause of cancer deaths worldwide[1]. Although therapeutic strategies have markedly improved clinical outcomes for BC patients, a substantial proportion of cases still eventually progress to metastatic disease, which continues to pose a formidable challenge to curative treatment. Indeed, a growing body of evidence indicates that BC is not merely a mass of neoplastic cells, but rather a complex ecosystem comprising the tumor microenvironment (TME) (Fig. 1). This TME is populated by a heterogeneous array of cell types, including endothelial cells, multiple stromal subsets, and immune cells. Among these immune components, macrophages exert a pivotal role in driving BC growth and progression. It is critical to emphasize, however, that this central regulatory function is typically acquired only after circulating monocytes are recruited to the tumor niche, differentiate into macrophages, and subsequently undergo further polarization and infiltration events.
In vitro studies utilizing murine RAW 264.7 macrophages and human THP-1 macrophages have provided robust mechanistic insights into this polarization process. Both M1 polarized macrophage and M2 polarized macrophage subsets contribute extensively to key hallmarks of tumorigenesis, including cancer cell proliferation, angiogenesis, and invasion into adjacent normal tissues. Specifically, M1-type macrophages (CD68 macrophage) have inflammatory and anti-tumor effects, while M2-type macrophages(CD163 macrophage) have immunosuppressive effects and exhibit pro-tumor activity[2].
This article reviews the role of macrophages in breast cancer, the divergent impacts of M1 versus M2 macrophages on breast cancer, the underlying mechanisms by which macrophages promote breast cancer metastasis, the interaction between macrophages and other immune cells in breast cancer, and the correlation between macrophage infiltration and breast cancer prognosis.
Table of Contents
1. The role of macrophages in breast cancer
2. Impact of M1 vs M2 macrophages on breast cancer
3. Mechanisms by which macrophages promote breast cancer metastasis
4. Interaction between macrophages and other cells in breast cancer
5. Macrophage infiltration and breast cancer prognosis
01 The role of macrophages in breast cancer
Macrophages have long been characterized as phagocytes and tumor cell destroyers, with phagocytic clearance of cancer cells being a well-documented canonical function of theirs[3]. However, as one of the most abundant immune cell populations within the TME of solid malignancies, macrophage infiltration correlates strongly with impaired overall survival across the majority of cancer types. Notably, the functional duality of macrophages in tumor biology is closely linked to their polarization into M1 and M2 macrophages, which underlies the complexity of macrophage function in immune system. Macrophages exert functional activity throughout all stages of tumor progression: at the primary tumor site, they drive angiogenesis, tumor cell invasion, and intravasation into the circulatory system; at distant metastatic loci, macrophages and their monocytic precursors orchestrate the formation of a pre-metastatic niche that conditions the local microenvironment for engraftment of incoming disseminated tumor cells (DTCs). Subsequently, these cells promote DTC extravasation and survival by suppressing immune-mediated clearance and directly activating pro-survival signaling pathways in tumor cells. Furthermore, macrophages sustain the outgrowth of disseminated tumor cells at metastatic sites by organizing the assembly of a supportive metastatic niche.
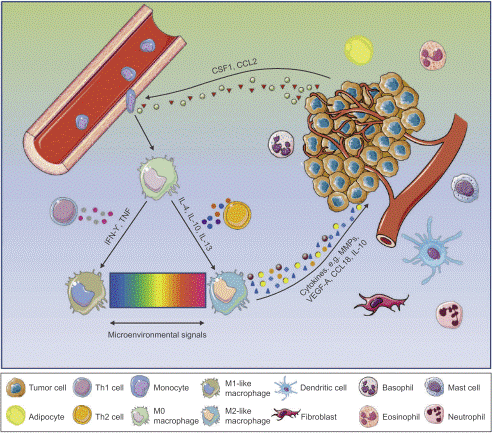
Fig. 1 The tumor microenvironment of breast cancer[4].
02 Impact of M1 vs M2 macrophages on breast cancer
Macrophages are innate immune cells and play a myriad of important roles such as host defense, tissue homeostasis, and modulating inflammatory responses[5]. To perform these functions, immature macrophages with high plasticity respond to microenvironmental cues, adopting a spectrum of effector phenotypes., among which M1-like and M2-like represent extreme polarization states[6] (Fig. 2). Classically activated M1 macrophages exhibit pro-inflammatory behavior by migrating to inflamed tissues, targeting pathogens with the production of reactive oxygen species (ROS), and having high antigen-expressing potential[7].These macrophages can be potent effector cells that kill tumor cells and can recruit cytotoxic T lymphocytes (CTLs) to activate adaptive immune responses. On the opposite side of the macrophage polarization spectrum, alternatively activated M2 macrophages secrete anti-inflammatory cytokines to induce immune tolerance and attract T regulatory cells (Tregs) and T helper 2 (Th2) cell subsets capable of protective type 2 responses but devoid of cytotoxic functions. M2 macrophages facilitate canonical tissue repair functions and in cancer are regarded as pro-tumor where they promote tissue remodeling and repair, stimulate angiogenesis with vascular endothelial growth factor (VEGF), and encourage tissue growth with transforming growth factor beta (TGF-β)[8].
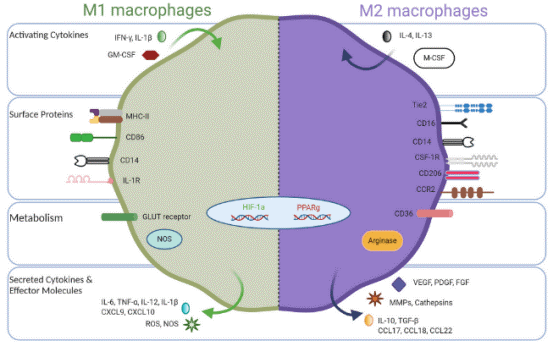
Fig. 2 Opposing phenotypes of M1-like and M2-like tumor-associated macrophages[9].
03 Mechanisms by which macrophages promote breast cancer metastasis
Breast cancer metastasis is a highly coordinated cascade process, in which macrophages play a central role that evolves from an "accomplice" to a "guide" and ultimately to a "guardian". This functional evolution is closely associated with the polarization of macrophages, a key feature in m1 m2 macrophage cancer research. Within the primary tumor, tumor cells secrete signals such as colony-stimulating factor 1 (CSF-1) and interleukin-4 (IL-4), which polarize macrophages into M2-type tumor-associated macrophages (TAMs) (a critical subset in tumor associated macrophage m1 m2 classification).These TAMs accumulate at the tumor invasive front and hypoxic regions, secreting potent enzymes including matrix metalloproteinases (MMPs) to degrade the extracellular matrix (ECM) and basement membrane, thereby establishing physical pathways for tumor cell dissemination. Meanwhile, TAMs release factors such as TGF-β and epidermal factor growth (EGF) that induce epithelial-mesenchymal transition (EMT) in cells, endowing tumor them with motility and invasiveness. Additionally, TAMs promote the formation of structurally disorganized and leaky neovessels by secreting VEGF and other pro-angiogenic factors; these neovessels act as "backdoors" that facilitate tumor cell intravasation into the circulatory system. More critically, even prior to the metastasis of the primary tumor, exosomes and soluble factors released by tumor cells mobilize bone marrow-derived cells to form "pre-metastatic niches" in distant organs (e.g., lungs, liver, and bones), creating an inflammatory and supportive microenvironment that "prepares the soil" for circulating tumor cells (CTCs). Once CTCs survive in the bloodstream and reach distant organs, resident macrophages and their derived cells strongly suppress the function of CTLs by expressing programmed death-ligand 1 (PD-L1) and secreting interleukin-10 (IL-10), enabling metastatic cells to evade immune surveillance and clearance. Finally, they continuously secrete growth factors such as EGF and platelet-derived growth factor (PDGF) and activate signaling pathways like STAT3, directly stimulating tumor cell proliferation and maintaining their stem cell characteristics, thus ensuring the survival and proliferation of micrometastases and eventually leading to the formation of life-threatening clinical metastases. It can be said that macrophages are deeply involved in the entire metastasis process, from local invasion, vascular permeation, distant colonization to final growth[10].
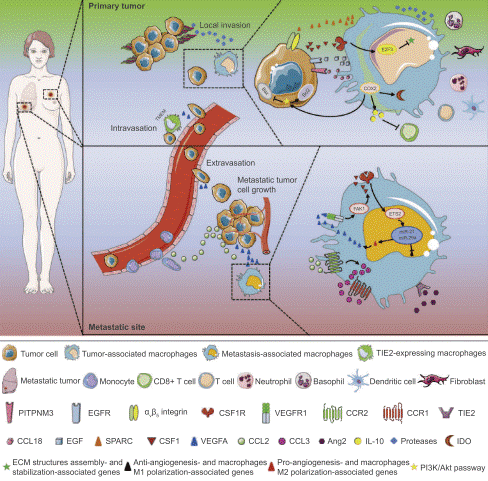
Fig. 3 Mechanisms of TAMs in promoting breast tumor growth and metastasis[4].
04 Interaction between macrophages and other cells in breast cancer
In cancer, TAMs are intricately involved in tumor biology by mediating tumor growth and progression, as well as contributing to therapy resistance. In breast cancer, TAMs can be highly abundant, accounting for over 50% of the total cellular composition within the tumor microenvironment. The breast cancer microenvironment also comprises fibroblasts, adipocytes, and multiple leukocyte subsets, which including neutrophils, lymphocytes, and dendritic cells. The accumulation of TAMs in breast cancer is sustained by resident macrophages and the recruitment of circulating monocytes.
In breast cancer subtypes such as triple-negative breast cancer (TNBC), TAMs can drive the differentiation of B cells into regulatory B cells (Bregs) via the secretion of factors including IL-10. These Bregs, in turn, exert potent immunosuppressive effects within the tumor microenvironment[11].TGF-β secreted by TAMs can significantly inhibit the activation, cytotoxicity (e.g., granzyme B secretion), and IFN-γ producing capacity of natural killer (NK) cells, thereby impairing the surveillance and tumoricidal activities of the innate immune system against tumor cells[12].TAMs secrete TGF-β and PDGF, which serve as key signals that activate quiescent fibroblasts and induce their transformation into tumor-promoting cancer-associated fibroblasts (CAFs). Conversely, activated CAFs secrete chemokine (C-X-C motif) ligand 12 (CXCL12), interleukin-6 (IL-6), and other soluble factors, which further recruit TAMs and sustain their M2-like pro-tumor phenotype[13].TAMs accumulate in poorly vascularized or hypoxic regions, where they secrete abundant potent pro-angiogenic factors, including vascular endothelial growth factor-A (VEGF-A), fibroblast growth factor 2 (FGF2), and matrix metalloproteinase 9 (MMP9). These factors stimulate the proliferation and migration of endothelial cells, ultimately driving the formation of neovessels with structural disorganization and functional abnormalities[14]. Within the TME, adipocyte, particularly those in close proximity to cancer cells, are induced to differentiate into cancer-associated adipocytes (CAAs). These CAAs engage in bidirectional crosstalk with TAMs: they supply metabolic energy to both TAMs and tumor cells, while concomitantly promoting local inflammation and driving the polarization of TAMs toward the M2 pro-tumor phenotype. In turn, TAMs secrete bioactive factors such as tumor necrosis factor-α (TNF-α), which modulate the metabolic activity and functional properties of adipocytes[15].
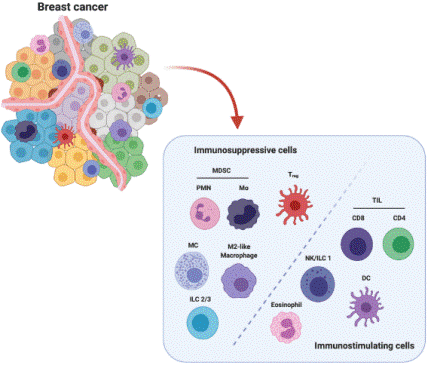
Fig. 4 Major players in breast cancer TME[16].
05 Macrophage infiltration and breast cancer prognosis
Despite prior evidence indicating that high infiltration of M1-like macrophages correlates with improved survival outcomes.On the other hand,high infiltration level of TAMs is associated with higher response rate to neoadjuvant chemotherapy(NAC)[17].Overall, high infiltration of M2-like tumor-associated macrophages (TAMs) serves as a robust adverse prognostic marker, as these cells actively drive tumor progression and immune evasion. In contrast, infiltration of M1-like macrophages correlates with enhanced anti-tumor immunity and improved patient survival outcomes. Notably, the predictive value of TAMs in neoadjuvant chemotherapy is highly contingent on their functional status and dynamic changes over time. Looking forward, the integration of macrophage quantity, phenotypic profile, and spatial distribution into a composite biomarker, coupled with the development of TAM-targeted therapeutic strategies, such as inhibiting macrophage recruitment, abrogating their tumor-promoting functions, or reprogramming these cells toward anti-tumor phenotypes, will hold profound clinical significance for ameliorating the prognosis of breast cancer patients.
In summary, macrophages are pivotal in breast cancer progression. M1 macrophages exert antitumor effects via pro-inflammatory and immune-activating functions, while M2 macrophages promote tumor growth and metastasis. TAMs facilitate metastasis through multiple cascades, including ECM degradation, EMT induction, pre-metastatic niche formation, and immune suppression. They interact with various immune (Tregs, NK cells) and stromal cells (CAFs, adipocytes) via cytokine signaling, forming pro-tumor loops.
Elabscience® Quick Overview of Popular Products
Table 1. Research Tools for breast cancer
|
Cat. No. |
Product Name |
|
E-BC-K138-F |
Reactive Oxygen Species (ROS) Fluorometric Assay Kit (Green) |
|
E-BC-F005 |
Reactive Oxygen Species (ROS) Fluorometric Assay Kit (Red) |
|
E-HSEL-H0002 |
High Sensitivity Human IL-2 (Interleukin 2) ELISA Kit |
|
E-EL-H0101 |
Human IL-4(Interleukin 4) ELISA Kit |
|
E-HSEL-H0003 |
High Sensitivity Human IL-6 (Interleukin 6) ELISA Kit |
|
E-OSEL-H0001 |
QuicKey Pro Human IL-6(Interleukin 6) ELISA Kit |
|
CQH001 |
CellaQuant™ Human IL-6 (Interleukin 6) ELISA Kit |
|
ESP-H0009S |
Human IL-6 (Interleukin 6) solid ELISPOT Kit |
|
E-HSEL-H0005 |
High Sensitivity Human IL-10 (Interleukin 10) ELISA Kit |
|
E-EL-H0109 |
Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit |
|
E-HSEL-H0007 |
High Sensitivity Human IFN-γ (Interferon Gamma) ELISA Kit |
|
E-EL-H0111 |
Human VEGF-A(Vascular Endothelial Cell Growth Factor A) ELISA Kit |
|
E-EL-H6075 |
Human MMP-9(Matrix Metalloproteinase 9) ELISA Kit |
|
E-AB-F1299L |
Elab Fluor® 488 Anti-Human CD68 Antibody[Y1/82A] |
|
E-AB-F1133C |
FITC Anti-Human CD274/PD-L1 Antibody[29E.2A3] |
|
XJH001 |
Human Th1/Th2 Flow Cytometry Staining Kit |
|
MIH008N |
EasySort™ Human Naïve CD8+T Cell Isolation Kit |
|
MIH003N |
EasySort™ Human CD8+ T Cell Isolation Kit |
|
MIH010N |
EasySort™ Human Memory CD8+T Cell Isolation Kit |
References:
[1] Azamjah, N., Y. Soltan-Zadeh, and F. Zayeri, Global trend of breast cancer mortality rate: a 25-year study. Asian Pacific journal of cancer prevention: APJCP, 2019. 20(7): p. 2015.
[2] Alves, A.M., L.F. Diel, and M.L. Lamers, Macrophages and prognosis of oral squamous cell carcinoma: A systematic review. Journal of Oral Pathology & Medicine, 2018. 47(5): p. 460-467.
[3] Qian, B.-Z. and J.W. Pollard, Macrophage diversity enhances tumor progression and metastasis. Cell, 2010. 141(1): p. 39-51.
[4] Qiu, S.-Q., et al., Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer treatment reviews, 2018. 70: p. 178-189.
[5] Wynn, T.A., A. Chawla, and J.W. Pollard, Macrophage biology in development, homeostasis and disease. Nature, 2013. 496(7446): p. 445-455.
[6] Gordon, S. and P.R. Taylor, Monocyte and macrophage heterogeneity. Nature Reviews Immunology, 2005. 5(12): p. 953-964.
[7] Haloul, M., et al., mTORC1-mediated polarization of M1 macrophages and their accumulation in the liver correlate with immunopathology in fatal ehrlichiosis. Scientific reports, 2019. 9(1): p. 14050.
[8] Jayasingam, S.D., et al., Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front Oncol, 2019. 9: p. 1512.
[9] Mehta, A.K., et al., Macrophage biology and mechanisms of immune suppression in breast cancer. Frontiers in immunology, 2021. 12: p. 643771.
[10] Obeid, E., et al., The role of tumor-associated macrophages in breast cancer progression (review). International Journal Of Oncology, 2013. 43(1): p. 5-12.
[11] Li, M., et al., B Cells in Breast Cancer Pathology. Cancers, 2023. 15(5): p. 1517.
[12] Xue, V.W., et al., Transforming growth factor-β: a multifunctional regulator of cancer immunity. Cancers, 2020. 12(11): p. 3099.
[13] Gunaydin, G., CAFs interacting with TAMs in tumor microenvironment to enhance tumorigenesis and immune evasion. Frontiers in oncology, 2021. 11: p. 668349.
[14] Dallavalasa, S., et al., The role of tumor associated macrophages (TAMs) in cancer progression, chemoresistance, angiogenesis and metastasis-current status. Current medicinal chemistry, 2021. 28(39): p. 8203-8236.
[15] Habanjar, O., et al., The impact of obesity, adipose tissue, and tumor microenvironment on macrophage polarization and metastasis. Biology, 2022. 11(2): p. 339.
[16] Salemme, V., et al., The Crosstalk Between Tumor Cells and the Immune Microenvironment in Breast Cancer: Implications for Immunotherapy. Frontiers in Oncology, 2021. 11.
[17] von der Lippe Gythfeldt H, Lien T, Tekpli X, et al. Immune phenotype of tumor microenvironment predicts response to bevacizumab in neoadjuvant treatment of ER‐positive breast cancer[J]. International Journal of Cancer, 2020, 147(9): 2515-2525.



