Identification Procedure of Mouse Regulatory T cell
Experimental Design
|
Cell
subpopulation
|
Species
|
Marker
|
Fluorescence
labeling
|
Clone
number
|
|
Treg
|
Mouse
|
CD4
|
FITC
|
GK1.5
|
|
CD25
|
APC
|
PC-61.5.3
|
||
|
Foxp3
|
PE
|
3G3
|
Flow Cytometry Antibodies Required for Mouse Regulatory T cell Identification
|
Marker
|
Fluorescence
|
Cat. No.
|
Specification
|
|
CD4
|
FITC
|
100T
|
|
|
CD25
|
APC
|
20T
|
|
|
Foxp3
|
PE
|
20T
|
Auxiliary Reagents Required for Mouse Regulatory T cell Identification
|
Purpose
|
Cat.
No.
|
Product
Name
|
Specification
|
|
Isotype control
|
APC Rat IgG1,
κ Isotype Control
|
20T
|
|
|
Isotype control
|
PE Mouse IgG1,
κ Isotype Control
|
20T
|
|
|
Cell staining buffer
|
Cell Staining Buffer
|
100 mL
|
|
|
Red blood cell lysate
|
10×ACK Lysis Buffer
|
100 mL
|
|
|
Fixation and Permeabilization buffer
|
Foxp3/Transcription
Factor Staining Kit
|
20 Assays
|
Panel Design
|
Purpose
|
Sample
|
Antibody
Collocation
|
|
Adjust the voltage
|
1
|
Black
|
|
Adjust compensation
|
2
|
CD4-FITC
|
|
3
|
CD25-APC
|
|
|
4
|
Foxp3-PE
|
|
|
APC-FMO in combination with Isotype Control for
auxiliary gating
|
5
|
CD4-FITC, Foxp3-PE; Rat IgG1, κ Isotype Control- APC
|
|
PE-FMO in combination with Isotype Control for
auxiliary gating
|
6
|
CD4-FITC, CD25-APC; Mouse IgG1, κ Isotype Control- PE
|
|
Full Panel
|
7
|
CD4-FITC, CD25-APC, Foxp3-PE
|
Experimental Procedure
Steps for isolating mouse spleen cells:
1)Take mouse spleen and place it in PBS.
2)Grind the spleen with a syringe plunger, use 70 μM cell filter for filtration and collect the filtrate into a 15 mL centrifuge tube.
3)Centrifuge at 300 ×g for 5 min, and obtain red cell precipitate.
4)Add 2 mL of 1× erythrocyte lysate to resuspend the cells. After lysing at room temperature for 2~3 minutes, immediately add 10 mL of PBS to terminate the lysis reaction.
5)Centrifuge the solution at 300 g for 5 minutes, discard the supernatant, and obtain red cell precipitate. Resuspend the spleen cells with cell staining buffer and perform cell counting, adjusting cell concentration to 1×107/mL.
Steps of Fixation and Permeabilization:
(The following are the experimental steps for the full staining tube. The Blank tube does not add antibodies, while the Single staining tube only adds corresponding Flow Cytometry antibodies in the corresponding steps. The Isotype control tube replaces the corresponding Flow Cytometry antibodies with the Isotype control antibody, and all tubes need to undergo Fixation and Permeabilization.)
1)Dilute Foxp3 detection specific Fixation and Permeabilization buffer based on the amount detected.
2)Take 100 μL mouse spleen cells1×106 cells) into an EP tube. Add 1 Test FITC Anti-Mouse CD4 (E-AB-F1097C) and 1Test APC Anti-Mouse CD25 (E-AB-F1102E), mixed. Incubated at 4 ℃ for 30 minutes.
3)After incubation, add 1 mL of Cell Staining Buffer, centrifuge at 300 × g for 5 minutes. Discard the supernatant and add 100 μL of Cell Staining Buffer to resuspend cells.
4)Add 1 mL of Fixation Working Solution, vortex mix well and react at 4℃ for 30 minutes. After the reaction is completed, centrifuge at 600 × g for 5 minutes and discard the supernatant.
5)Add 2 mL of Permeabilization Working Solution, vortex mix well and react at 4℃ for 30 minutes. After the reaction is completed, centrifuge at 600 × g for 5 minutes and discard the supernatant.
6)Repeat Step 5.
7)Add 100 μL of 1× Permeabilization Working Solution to resuspend the sample. Then add PE Anti-Mouse Foxp3 (E-AB-F1238D), mix well and react at RT for 30 minutes.
8)Add 2 mL of Permeabilization Working Solution, centrifuge at 600 × g for 5 minutes and discard the supernatant.
9)Add 200 μL cell staining buffer (E-CK-A107) to resuspend the sample. Conduct the detection.
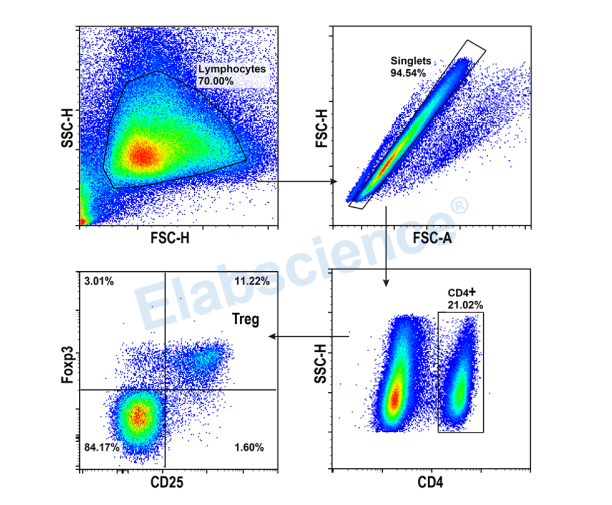
Experimental Result

Detection of Treg (3-color) in Mouse Spleen
Tips:
▶ There is fluorescence spillover, so it is necessary to set single positive tubes for compensation.
▶ CD4 cell population is obvious, and there is no need of Isotype Control. But CD25 and Foxp3 populations are not obvious, and Isotype Controls are needed.
▶ This experiment was validated using normal mouse spleen without any biological control.
▶ Gating:
1)Gating the target cells based on FSC-A and SSC-A.
2)Utilizing the light scattering characteristics to remove adhesions and obtain single cells.
3)Determine CD4+ Th cells through CD4.
4)Identifying Treg cell subpopulations through CD25+/Foxp3+.



