CD127 VS Foxp3
Although Foxp3 is currently recognized as the most specific indicator of regulatory T cells, its localization within the cell requires fixation of the cell and breaking of the nuclear membrane for staining. Therefore, this method is not suitable for isolating live Tregs. And this method is relatively traditional and the operation is relatively complex, and even a slight mistake can affect the experimental results.
CD127 belongs to the cell surface marker and does not require Fixation and Permeabilization. Therefore, the current mainstream direction is to detect human Tregs through CD127.
Discovery of CD127
CD127 is a new indicator discovered in 2006 for detecting CD4+CD25+regulatory T cells. Detection of CD127 does not require Fixation and Permeabilization, which is more convenient than Foxp3.
Research has found a negative correlation between the expression of CD127 and Foxp3 in CD4+CD25+purified cells. The number of CD4+CD25+T cells purified through CD4+CD127low is similar to that purified by Foxp3, and these cells exhibit typical characteristics of CD4+CD25+regulatory T cells.
CD4+CD25+CD127-/low is a more specific surface marker molecule for detecting Tregs than CD4+CD25+Foxp3+.
|
Cell
subpopulation
|
Species
|
Marker
|
Fluorescence labeling
|
Clone
number
|
|
Treg
|
Human
|
CD45
|
Elab Fluor® Violet 450
|
HI30
|
|
CD3
|
Elab Fluor® Red 780
|
OKT3
|
||
|
CD4
|
FITC
|
RPA-T4
|
||
|
CD8a
|
PerCP/Cyanine5.5
|
OKT-8
|
||
|
CD25
|
PE
|
BC96
|
||
|
CD127
|
Elab Fluor® 647
|
A019D5
|
Flow Cytometry Antibodies Required for Human Regulatory T cell Identification
|
Marker
|
Fluorescence
|
Cat. No.
|
Specification
|
|
Elab Fluor® Violet 450
|
20T
|
||
|
Elab Fluor® Red 780
|
20T
|
||
|
FITC
|
20T
|
||
|
PerCP/Cyanine5.5
|
20T
|
||
|
PE
|
20T
|
||
|
Elab Fluor® 647
|
20T
|
Auxiliary Reagents Required for Human Regulatory T cell Identification
|
Purpose
|
Cat. No.
|
Product
Name
|
Specification
|
|
Cell staining buffer
|
Cell Staining Buffer
|
100 mL
|
|
|
Red blood cell lysate
|
10×ACK Lysis Buffer
|
100 mL
|
Panel Design
|
Purpose
|
Sample
|
Antibody
Collocation
|
|
Adjust the voltage
|
1
|
Blank
|
|
Adjust compensation
|
2
|
CD45-Elab Fluor® Violet 450
|
|
3
|
CD3-Elab Fluor® Red 780
|
|
|
4
|
CD4-FITC
|
|
|
5
|
CD8a-PerCP/Cyanine5.5
|
|
|
6
|
CD25-PE
|
|
|
7
|
CD127-Elab Fluor® 647
|
|
|
Full Panel
|
8
|
CD45-Elab Fluor® Violet 450, CD3-Elab Fluor® Red 780,
CD4-FITC, CD8a-PerCP/Cyanine5.5, CD25-PE, CD127-Elab Fluor® 647
|
Experimental Procedure
1)Add 100 μL of fresh blood and 2 mL of 1× ACK red blood cell lysate to the centrifuge tube, mix and lyse at 4°C for 10 min.
2)Centrifuge at 300 g for 5 min (centrifuge immediately after lysis to prevent cells damage), discard the supernatant, obtain white cell pellet.
3)Add 100 μL of PBS to resuspend the cells. Add 1 Test corresponding Flow Cytometry antibody and mix well. React at RT for 30 minutes.
4)Add 200 μL cell staining buffer (E-CK-A107), centrifuge at 300 × g for 5 minutes and discard the supernatant. Add 200 μL cell staining buffer (E-CK-A107) to resuspend the sample.
5)Conduct the detection.
Experimental Result
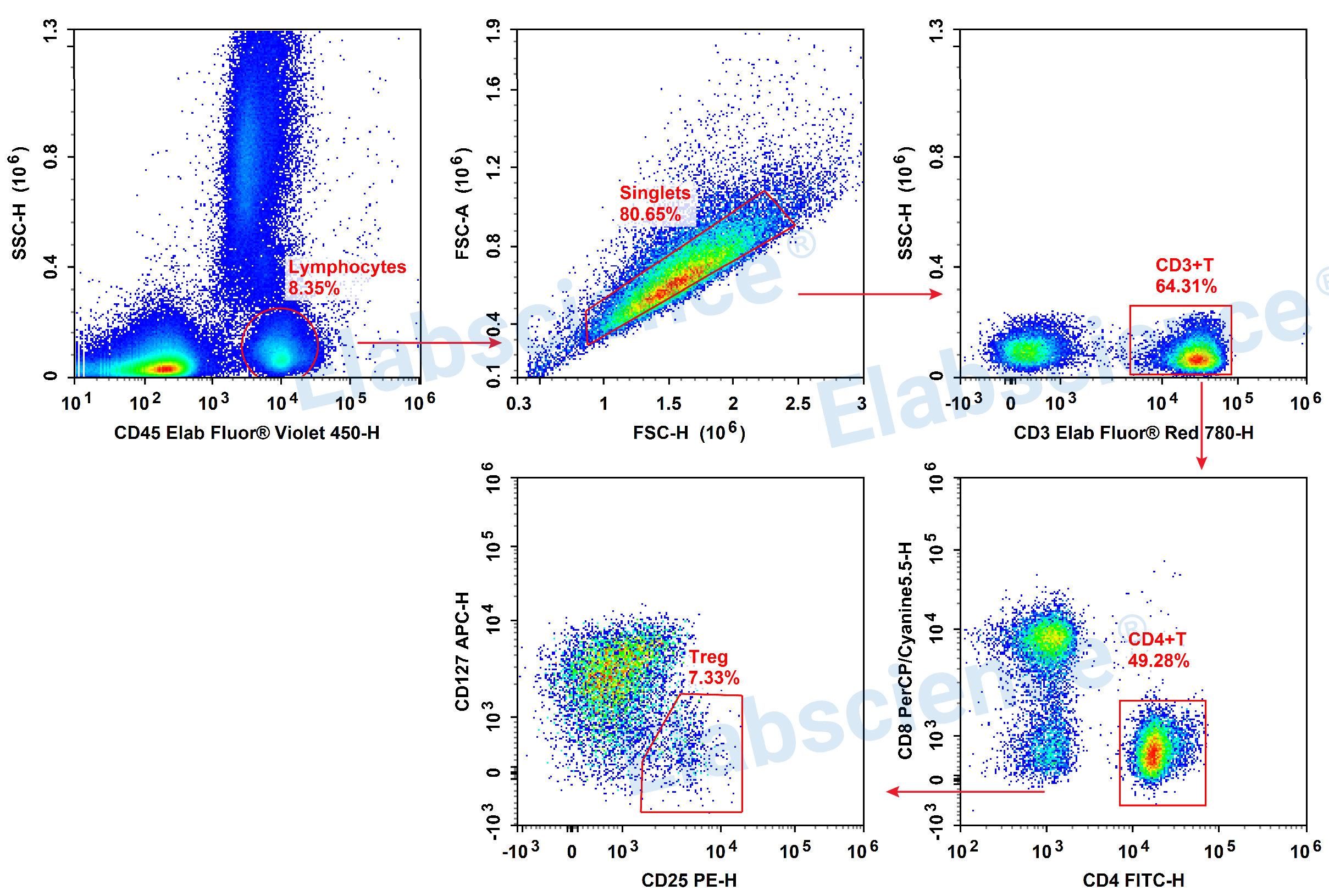
Detection of Treg (6-color) in Human Peripheral Blood
Tips:
▶ There is fluorescence spillover, so it is necessary to set single positive tubes for compensation.
▶ This experiment was validated using normal human peripheral blood without any biological control.
▶ Increase CD45 indicators to facilitate gating lymphocytes.
▶ Gating:
1)The lymphocyte can be gated directly through CD45 and SSC
2)Utilizing the FSC-H and SSC-H to remove adhesions and obtain single cells.
3)Determine CD3+ T cells through CD3.
4)Determine CD4+ Th cells through CD4 and CD8.
5)Identifying Treg cell subpopulations through CD25+CD127-/low.



