Neuroimmunology investigates the essential interactions between the nervous and immune systems, with brain-resident and recruited immune cells playing critical roles in maintaining neurological health and driving disease. Once considered an immune-privileged site, the brain is now recognized as a venue for active and indispensable immune activity that influences development, homeostasis, and pathology[1].
This review outlines the key immune cells within the brain, including microglia, astrocytes, peripherally derived populations, and their homeostatic functions. It subsequently examines how dysregulation of these cells, particularly microglia and astrocytes, contributes to neuroinflammation and neurodegeneration. Furthermore, it analyzes the mechanisms by which peripheral immune cells infiltrate the central nervous system via the blood brain barrier and their pathological roles in autoimmune conditions and chronic neuroinflammatory states. Ultimately, this review systematically elucidates the pivotal role of neuroimmune interactions in the pathogenesis and progression of neurological disorders.
Table of Contents
1. Neuroimmunology: the intersection of the nervous system and the immune system in the brain
2. What are the key immune cells in the brain and their functions?
3. How do microglia contribute to neurological diseases?
4. Astrocytes and neuroinflammation in disease
5. The role of peripheral immune cells in brain health and disease
01 Neuroimmunology: the intersection of the nervous system and the immune system in the brain
Neuroimmunology is an interdisciplinary field dedicated to investigating the complex, bidirectional interactions between the nervous and immune systems. Historically, the brain was considered an “immune-privileged” site, however, research over the past two decades has fundamentally redefined this view. It is now recognized that the brain constitutes a dynamic and actively surveyed immune environment, where neural and immune cells engage in continuous crosstalk via shared signaling pathways to maintain homeostasis. Dysregulation of this precise dialogue is a core mechanism underlying numerous neurological disorders[1,2].
The early concept of “central nervous system (CNS) immune” privilege originated from the observation that tissues transplanted into the brain parenchyma are not easily rejected. This was attributed to three key anatomical features: the blood brain barrier (BBB, which restricts the passage of cells and molecules), the absence of classical lymphatic vessels (which limits antigen drainage), and the limited function of antigen-presenting cells within the brain. These anatomical features supported the long-held view of CNS isolation from peripheral immunity[2,3].
However, contemporary research has elucidated that the CNS maintains precise, albeit specialized, immune surveillance through dedicated border compartments. These anatomical interfaces, collectively conceptualized as part of a broader “neuro-immune connectome” governing body-wide interactions, are essential for understanding neuroimmunological disease. Key CNS border compartments include:
The Meninges: This three-layered membrane enveloping the brain functions as an immunologically active hub, populated by diverse immune cells (e.g., T cells, B cells, macrophages). Meningeal immune cells release signaling molecules that can directly modulate cortical neuronal activity and behavior[2,3,4].
The Choroid Plexus: A vascular structure vital for cerebrospinal fluid (CSF) production, serving as a selective gateway between peripheral blood and the CSF compartment[2].
Perivascular Spaces: Fluid-filled channels surrounding cerebral vessels that facilitate the exchange of interstitial fluid and CSF, enabling the trafficking of antigens and immune cells[2].
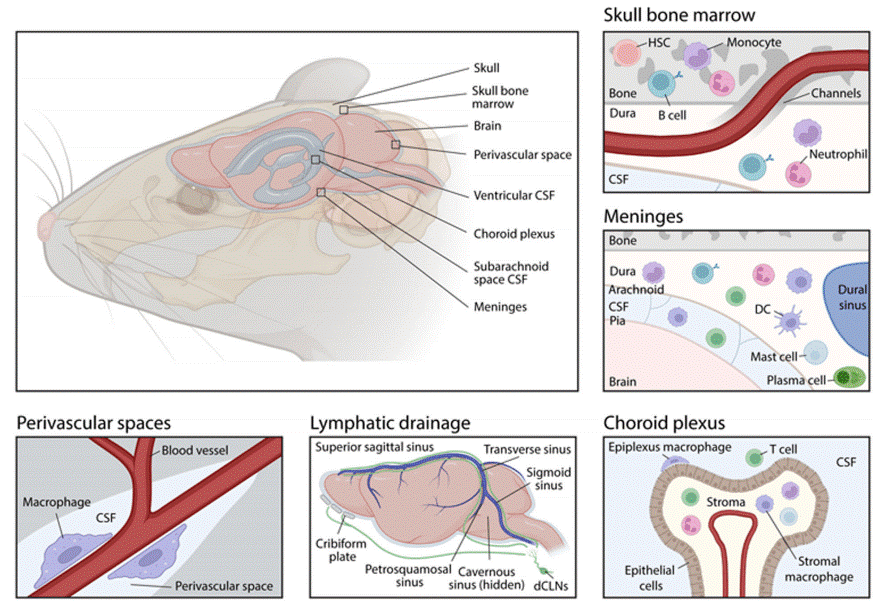
Fig. 1 Anatomical sites for neuroimmune interactions at the brain borders[2].
02 What are the key immune cells in the brain and their functions?
The central immune cells in the brain are microglia, astrocytes, oligodendrocytes, and perivascular macrophages, each playing distinct and complementary roles in maintaining neural homeostasis and responding to pathological conditions.
2.1 Microglia
Microglia (microglial cells) are the primary innate immune cells of the central nervous system (CNS). Originating from progenitors in the yolk sac during embryogenesis, they populate the brain early in development. Their key functions include:
Immune Surveillance: Constantly monitoring the CNS environment, microglia detect pathogens, damaged cells, and molecular signals of distress.
Phagocytosis: Microglia act as “cleaners”, engulfing cellular debris, apoptotic neurons, or misfolded proteins linked to neurodegenerative diseases like Alzheimer's disease (AD)[5,6].
Neuroinflammation: Activating either pro-inflammatory or anti-inflammatory states (termed M1 and M2 phenotypes), microglia mediate inflammatory responses. This dual role allows them to protect against acute damage but may contribute to chronic neurodegeneration if dysregulated[7,8].
Role in Development: Microglia are key facilitators of synaptic pruning during brain development, ensuring proper neuronal connectivity[9].
2.2 Astrocytes
Astrocytes are the most abundant glial cells in the brain. While their traditional role centers on supporting neurons, emerging evidence highlights their dynamic immune functions:
Blood brain barrier (BBB) Regulation: Astrocytes are critical in maintaining BBB integrity by interacting with endothelial cells and reducing CNS vulnerability to peripheral immune infiltration[10].
Neuroinflammation Modulation: They release signaling molecules like cytokines and chemokines under stress or injury, which can amplify or resolve local inflammation[11,12].
Synaptic Regulation: Astrocytes regulate neuronal activity by controlling neurotransmitter uptake and recycling, but inflammatory astrocytes (A1 phenotype) have been implicated in neuronal dysfunction during conditions like AD and traumatic brain injury (TBI)[12.13].
Neurovascular Interactions: Collaboration between astrocytes and microglia drives responses to metabolic stresses and ischemic events[8].
2.3 Oligodendrocytes
Oligodendrocytes are responsible for myelin production, which insulates axons, ensuring efficient signal conduction. Beyond their structural role, they perform several immune-related tasks:
Immune Modulation: Recent research identifies oligodendrocytes as active participants in local immune responses, particularly in modulating inflammation near damaged myelin or brain lesions[14].
Response to CNS Injury: Upon activation, oligodendrocytes emit anti-inflammatory signals, possibly aiding neural repair during neurodegenerative diseases like multiple sclerosis[15].
2.4 Perivascular Macrophages
These immune cells are located along the blood vessels of the CNS, particularly at the BBB. Their main roles include:
Antigen Presentation: Perivascular macrophages help activate adaptive immune responses by presenting antigens to T cells trafficked through the CNS.
Immune Surveillance: They maintain watch over the neurovascular environment, identifying potential pathogens or systemic immune signals[16].
Microglia, astrocytes, oligodendrocytes, and perivascular macrophages do not function in isolation but engage in critical crosstalk that modulates neuroinflammation and homeostasis. Key interactions include: the induction by microglia of neurotoxic A1 astrocyte polarization, exacerbating neurodegeneration; the collaboration between oligodendrocytes and microglia to repair demyelinated axons; and the cooperative work of astrocytes with perivascular macrophages to precisely regulate blood brain barrier (BBB) permeability during neuroinflammation[8,10,14].
In summary, these key neuroglial and immune cells, such as microglia (microglial cells), astrocytes, oligodendrocytes, and perivascular macrophages, serve as indispensable players in maintaining brain health, responding to injury, and regulating neuroinflammation. Their intricate interconnections and dual roles (either protective or detrimental) underscore the intricate nature of immune regulation within the central nervous system (CNS).
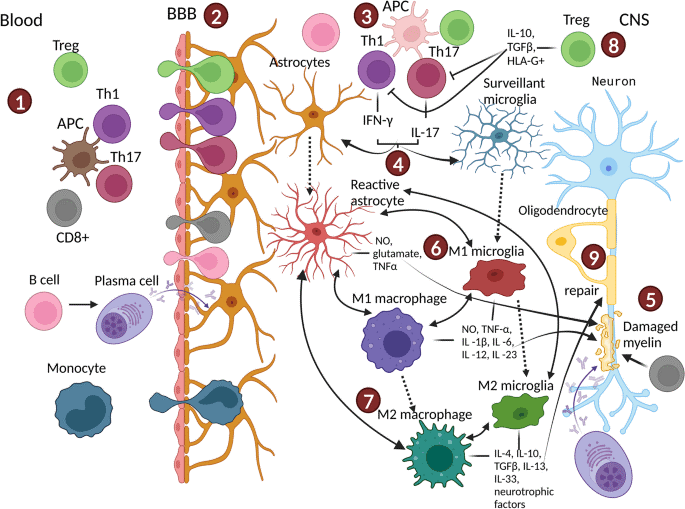
Fig. 2 The central immune cells in the brain[11].
03 How do microglia contribute to neurological diseases?
Microglia (microglial cells), as the central nervous system's resident immune cells, are critical players in maintaining brain homeostasis and responding to pathological insults. Their role in neurological diseases encompasses both protective and damaging processes, making them dual contributors to disease progression and resolution.
3.1 Protective Roles in Neurological Health
Microglia play essential roles in maintaining neural network functionality and responding to CNS insults. As the primary line of immune defense against infections, they eliminate pathogens, debris, and apoptotic cells through phagocytosis (microglia phagocytosis). In terms of homeostasis regulation, microglia contribute to neural plasticity and development across critical periods and lifespan by pruning synapses and removing redundant neural networks. Additionally, under mild stress or damage, microglia switch to an anti-inflammatory (M2) phenotype, secrete neuroprotective cytokines, and facilitate tissue repair, thereby exerting neuroprotective effects[28,29].
3.2 Pathological Roles in Neurological Diseases
While microglia are indispensable for brain health, their dysregulated activation leads to the pathogenesis of many neurological disorders. This process is often mediated by pro-inflammatory signaling, chronic activation, or impaired functionality[28].
Neurodegeneration and Microglial Overactivation
In neurodegenerative diseases including Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis (ALS), microglial overactivation frequently exacerbates pathological progression by releasing pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α[30,37]. Key underlying mechanisms involve three core aspects: first, chronically activated microglia (predominantly the M1 phenotype) perpetuate neural tissue damage through excessive inflammatory responses, secreting neurotoxic factors like reactive oxygen species (ROS) and nitric oxide; second, while microglia play a critical role in clearing pathological aggregates (e.g., amyloid-beta plaques and hyperphosphorylated tau tangles, the hallmark lesions of Alzheimer's disease), inefficiencies in this clearance process coupled with pro-inflammatory signaling further aggravate neurodegeneration; third, dysregulated synaptic pruning by overactivated microglia contributes to synaptic loss and functional decline, a phenomenon observed in conditions such as Alzheimer's disease and schizophrenia[28,30].
Microglial Polarization and Dual Roles
Microglia undergo dynamic phenotypic switching between pro-inflammatory (M1) and anti-inflammatory (M2) states in response to environmental cues: the M1 phenotype promotes neuroinflammation and neurodegeneration via the release of pro-inflammatory cytokines and chemokines, while the M2 phenotype enhances neuroprotection, tissue repair, and clearance of pathological aggregates. Dysregulation of this M1/M2 balance serves as a key driver of various neurological conditions, including multiple sclerosis, traumatic brain injury, and stroke[29,31].
Neuroinflammation and Blood–Brain Barrier (BBB) Dysfunction
Microglia-mediated neuroinflammation is a primary contributor to BBB breakdown, allowing harmful peripheral immune cells and substances to infiltrate the CNS. This disruption fuels disease progression by amplifying neuroinflammation and accelerating neuronal damage in diseases like Alzheimer's and Parkinson's[29].
Contribution to Neurodevelopmental Disorders
Microglial dysfunction is increasingly implicated in neurodevelopmental disorders such as autism spectrum disorder (ASD). Aberrant microglial activity during development may impair synapse refinement and contribute to the pathogenesis of ASD-associated neuroinflammation[32].
3.3 Emerging Understanding and Therapeutic Insights
Recent studies have provided transformative insights into microglial biology and their therapeutic potential for neurological disorders. Key strategies involve modulating microglial polarization toward the neuroprotective M2 phenotype or suppressing hyperactive microglia, both of which show promise for treating neurodegenerative diseases. Further approaches include deciphering the transcriptional and epigenetic mechanisms that govern microglial activation to identify novel therapeutic pathways, developing engineered microglia-based cell therapies to enhance the clearance of pathological aggregates and regulate neuroinflammation, and exploring nutraceuticals, such as green tea polyphenols and spirulina, as adjunctive treatments to attenuate microglia-mediated neuroinflammation[29,33].
Microglia are deeply intertwined with neurological disease processes, acting as double-edged swords capable of neuroprotection and neurotoxicity. Their phenotypic and functional plasticity, coupled with their central role in neuroinflammation, synaptic pruning, and aggregate clearance, highlights them as a promising therapeutic target in neurodegenerative and neurodevelopmental conditions. Extensive research continues to unravel their complex functions and offers hope for novel interventions.
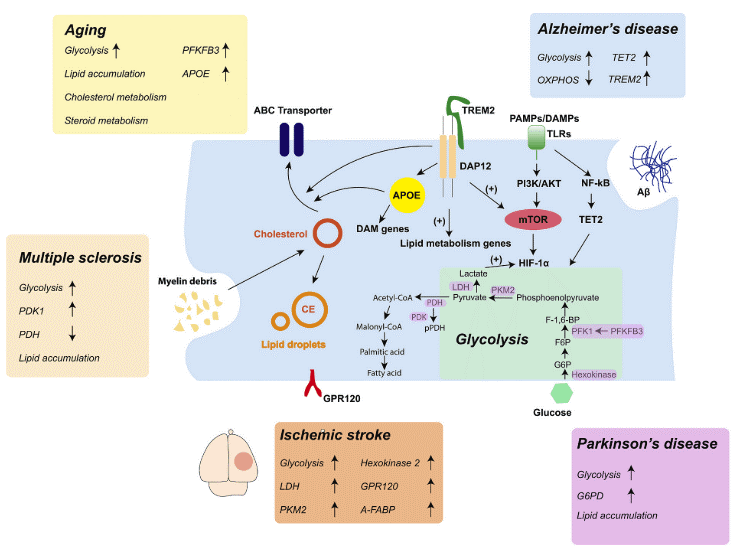
Fig. 3 Microglia metabolic reprogramming and related signaling pathways in different CNS diseases[37].
04 Astrocytes and neuroinflammation in disease
Astrocytes play a pivotal role in the regulation of neuroinflammation, a central process in various neurological and neurodegenerative disorders. These glial cells, the most abundant in the central nervous system (CNS), display a remarkable functional and phenotypic plasticity that allows them to participate actively in both the initiation and resolution phases of neuroinflammation. Their roles in this context are diverse and can be neuroprotective or neurotoxic depending on the specific molecular signals and cellular interactions present within the CNS.
4.1 Astrocytic Mechanisms in Neuroinflammation
Reactive Astrogliosis: Upon exposure to central nervous system (CNS) injury or pathological insults, astrocytes undergo a phenotypic transformation termed reactive astrogliosis. This process is characterized by morphological and functional remodeling, encompassing cellular hypertrophy, enhanced proliferative capacity, and distinctive shifts in gene expression profiles. These adaptive changes equip astrocytes to mount targeted responses to tissue damage and precisely regulate local inflammatory cascades[17]. Notably, reactive astrocytes exhibit functional dichotomy, adopting either a neuroprotective (A2 phenotype) or neurotoxic (A1 phenotype) role. A2-phenotype astrocytes promote tissue repair by secreting neuroprotective factors and facilitating regenerative microenvironments, whereas A1-phenotype astrocytes, typically induced by pro-inflammatory activated microglia, release pro-inflammatory mediators and cytotoxic substances that contribute to neuronal dysfunction and synaptic impairment[18].
Release of Cytokines and Chemokines: Astrocytes serve as pivotal sources of both pro-inflammatory and anti-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-10 (IL-10). These signaling molecules orchestrate the spatiotemporal recruitment and activation of immune cells, such as resident microglia, infiltrating peripheral macrophages, and T lymphocytes, thereby shaping the amplitude and duration of inflammatory responses within the CNS[17,19]. Sustained overexpression of pro-inflammatory cytokines by astrocytes is a hallmark of chronic neuroinflammation, and this aberrant secretion has been mechanistically linked to the pathogenesis of diverse neurodegenerative and neuroinflammatory disorders, including multiple sclerosis (MS), Alzheimer’s disease (AD), and Parkinson’s disease (PD)[20].
Astrocyte-Immune Cell Interactions: Via direct cell-cell contact and paracrine signaling crosstalk, astrocytes maintain intimate functional interactions with microglia, the primary resident immune cells of the CNS. This bidirectional interplay either amplifies inflammatory cascades or dampens them in a context-dependent manner. For instance, toll-like receptor (TLR)-mediated astrocyte-microglial crosstalk has been implicated in both protective immune surveillance and detrimental inflammatory responses in neurodegenerative conditions[17,21]. Astrocytes also modulate the activity of CNS-infiltrating lymphocytes (e.g., CD4+ T cells in MS). They secrete chemokine gradients to guide targeted immune cell recruitment and express surface molecules that fine-tune lymphocyte activation and effector functions, thereby integrating peripheral immune signals into CNS inflammatory networks[22].
Oxidative Stress and Nitrosative Stress: Astrocytes are integral to the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) during neuroinflammatory episodes. While ROS and RNS play essential roles in pathogen clearance and innate immune defense, their sustained overproduction disrupts redox homeostasis, triggering oxidative and nitrosative damage to neurons, glial cells, and the blood brain barrier (BBB). This cumulative damage exacerbates neurodegenerative processes and perpetuates the inflammatory cycle in pathological contexts[23].
4.2 Pathological Implications of Astrocyte-Mediated Neuroinflammation
Across diverse neurological conditions, astrocytes exert pivotal yet context-dependent roles in disease progression. In traumatic brain injury (TBI), they drive secondary injury via inflammatory mediator release and BBB disruption, with glial scar formation limiting damage spread but impeding CNS regeneration[24]. In neurodegenerative diseases, astrocytes contribute to Alzheimer’s disease pathogenesis through interactions with amyloid-beta plaques, modulation of tau pathology, and pro-inflammatory cytokine secretion; in Parkinson’s disease, mutations like LRRK2 (G2019S)-mediated astrocytic dysfunction impairs synaptic homeostasis and amplifies TNF-α-dependent inflammatory signaling[25,26]. During multiple sclerosis (MS) and its experimental model experimental autoimmune encephalomyelitis (EAE), astrocytes act as antigen-presenting cells to facilitate immune cell infiltration, promote demyelination, induce axonal damage, and enhance excitotoxicity via glutamate release. Additionally, aberrant activation of hippocampal astrocytes disrupts synaptic plasticity and cognitive function, driven by neurotoxic cytokine secretion and impaired neuromodulatory support under chronic inflammation[22,26].
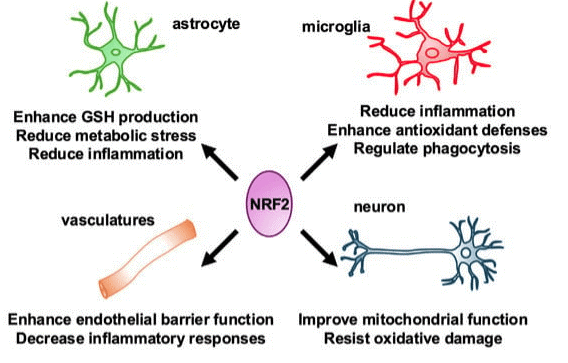
Fig. 4 The influence of Nrf2 on the types and structures of microglia and astrocytes in the nervous system[25].
4.3 Therapeutic Approaches Targeting Astrocytes
Given the dual roles of astrocytes in neuroprotection and neurodegeneration, therapeutic strategies are centered on modulating their activity to balance these contrasting effects. Anti-inflammatory therapies utilize pharmacological agents targeting pathways like NF-κB and JAK-STAT to reduce the production of neurotoxic pro-inflammatory mediators by astrocytes. Epigenetic modulation strategies aim to mitigate chronic neuroinflammation by targeting the inflammatory epigenetic memory of astrocytes, with novel approaches involving regulators such as histone deacetylase inhibitors. Metabolic interventions, particularly those targeting astrocyte-specific pathways like PHGDH-mediated serine synthesis, have shown potential in alleviating neuroinflammation and supporting neuronal survival in animal models of neurodegeneration. Additionally, emerging astrocyte-specific gene therapies, leveraging adeno-associated virus (AAV) vectors, are being explored, for example, inhibiting A1-like astrocytic phenotypes via such genetic approaches could limit neurotoxicity in neurodegenerative diseases[25,27].
Astrocytes are indispensable regulators of neuroinflammation, with multifaceted roles in maintaining CNS homeostasis, but they also contribute significantly to the pathophysiology of numerous neurological disorders when dysregulated. Understanding their mechanisms of action, interaction networks, and phenotypic diversity is fundamental for developing targeted therapies that leverage their protective functions while mitigating their deleterious effects.
05 The role of peripheral immune cells in brain health and disease
Peripheral immune cells, including T cells, B cells, macrophages, dendritic cells (DCs), and neutrophils, play pivotal roles in the development, progression, and resolution of various disease states, exerting a profound impact on brain health through complex bidirectional communication with the central nervous system (CNS). This crosstalk is critical for maintaining cerebral homeostasis, mediating injury responses, regulating neuroinflammatory processes. Here, we detail the multifaceted roles of peripheral immune cells in CNS physiology and pathology, with a focus on their interaction mechanisms with the brain.
5.1 Peripheral Immune Cells in Brain Homeostasis and Pathological Perturbations
Under physiological conditions, peripheral immune cells contribute to brain health via immune surveillance, synaptic maintenance, trophic support, and anti-inflammatory effects. For instance, regulatory T cells (Tregs) modulate microglial activity to preserve CNS immune homeostasis and regulate synapse stability, thereby influencing learning and memory. Peripherally recruited monocytes and macrophages secrete trophic factors such as brain-derived neurotrophic factor (BDNF) to support neuronal function and produce anti-inflammatory cytokines (e.g., IL-10) that facilitate tissue repair post-injury.
Conversely, chronic systemic inflammation, triggered by infections or metabolic disorders, can aberrantly activate peripheral immune cells, which release pro-inflammatory cytokines (e.g., IL-6, TNF-α) to perturb CNS function, potentially impairing cognitive and emotional regulation. These cells also exhibit context-dependent dual roles in the pathogenesis of CNS-related diseases:
In Alzheimer’s disease (AD), T cells, monocytes, and macrophages may facilitate amyloid-beta (Aβ) plaque clearance but can exacerbate neuronal damage and cognitive decline via chronic aberrant activation[25,26].
In Parkinson’s disease (PD), infiltrating CD4+ T cells induce microglial activation, leading to the release of reactive oxygen species (ROS) and pro-inflammatory cytokines that aggravate neuronal loss in the striatum and substantia nigra[22,23].
In stroke, neutrophils and other immune subsets expand infarct volume during the acute phase, whereas M2-polarized macrophages and Tregs mediate anti-inflammatory responses and promote tissue repair in the recovery phase.
In mood disorders such as depression, peripheral pro-inflammatory cytokines (e.g., TNF-α) traverse the compromised blood brain barrier (BBB), disrupt neurotransmitter homeostasis, and contribute to disease pathogenesis[34].
5.2 Mechanisms Underlying Peripheral Immune Cell-CNS Crosstalk
The interaction between peripheral immune cells and the brain is mediated by intricate anatomical pathways, molecular signaling networks, and cellular crosstalk, which collectively regulate physiological homeostasis and pathological responses:
1) Anatomical Routes for Immune Communication
The BBB normally restricts immune cell entry, but its permeability increases under neuroinflammatory conditions, enabling T cells, monocytes, and neutrophils to infiltrate the brain parenchyma and activate resident glial cells. Circumventricular organs (CVOs), such as the subfornical organ and median eminence, lack a complete BBB and permit direct immune signal exchange between the periphery and CNS. Additionally, the glymphatic system functions as a lymphatic-like clearance pathway, facilitating the transport of immune mediators and cerebrospinal fluid exchange to modulate neuroinflammation and brain homeostasis[10,14,23].
2) Molecular Signaling Mediators
Soluble factors, primarily cytokines and chemokines, drive peripheral immune cell-CNS communication. Pro-inflammatory cytokines (e.g., IL-6, TNF-α) released during systemic inflammation enhance BBB permeability and promote immune cell recruitment[30,36]. Chemokines such as CCL2 and CXCL10 further guide the directional migration of these cells into the CNS. Hormonal signals, including stress-induced glucocorticoids, and sympathetic nervous system activity via adrenergic receptors also critically regulate this neuroimmune crosstalk under inflammatory or pathological states[30,36].
3) Cellular Crosstalk in Neuroimmune Responses
In neuroimmune responses, CNS-resident cells (e.g., microglia, astrocytes) engage in intimate interactions with infiltrating peripheral immune cells. Microglia, as resident immune cells, can be activated by peripheral cytokines, polarized into a proinflammatory phenotype, and thereby exacerbate neuronal damage in neurodegenerative lesions. Astrocytes amplify neuroinflammatory responses by releasing cytokines (e.g., IL-1β) and regulating BBB integrity. Meanwhile, infiltrating peripheral immune cells, including Tregs, Th1/Th17 effector T cell subsets, and monocyte-derived macrophages, collectively modulate the local immune microenvironment, exerting dual roles in promoting repair or aggravating damage depending on the pathological context[34].
4) Neural Pathways for Bidirectional Communication
Neural pathways, particularly the vagus nerve and its associated cholinergic pathways, serve as key conduits for bidirectional immune-brain communication. As a critical afferent pathway, the vagus nerve directly detects peripheral inflammatory signals and transmits this information to the brainstem, thereby regulating systemic immune responses. Conversely, neurotransmitters such as acetylcholine inhibit pro-inflammatory cytokine production by peripheral immune cells via efferent signals, enabling precise regulation of neuroimmune dynamics. This neuroimmunomodulatory circuit maintains the body’s homeostatic balance under inflammatory conditions[19,20,30].
Peripheral immune cells mediate a broad spectrum of physiological and pathological processes in the CNS through dynamic interactions with resident cells and neural and molecular networks. The balance between protective immune surveillance and detrimental immune dysregulation is critical for neuroprotection and the pathogenesis of CNS diseases. Understanding these intricate crosstalk mechanisms not only provides fundamental insights into brain-immune homeostasis but also paves the way for innovative therapeutic strategies targeting neuroinflammatory and neurodegenerative disorders.
References:
[1] Weiner, H. L. (2024). Immune mechanisms and shared immune targets in neurodegenerative diseases. Nature Reviews Neurology, 21(2), 67–85. https://doi.org/10.1038/s41582-024-01046-7.
[2] Rustenhoven J , Kipnis J .Brain borders at the central stage of neuroimmunology[J]. Nature[2025-12-02]. DOI:10.1038/s41586-022-05474-7.
[3] Louveau, A., Smirnov, I., Keyes, T. J., Eccles, J. D., Rouhani, S. J., Peske, J. D., Derecki, N. C., Castle, D., Mandell, J. W., Lee, K. S., Harris, T. H., & Kipnis, J. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature, 523(7560), 337–341. https://doi.org/10.1038/nature14432.
[4] Smyth, L. C. D., & Kipnis, J. (2025). Redefining CNS immune privilege. Nature Reviews Immunology, 25(10), 766–775. https://doi.org/10.1038/s41577-025-01175-0.
[5] Rajesh, Y., & Kanneganti, T.-D. (2022). Innate Immune Cell Death in Neuroinflammation and Alzheimer’s Disease. Cells, 11(12), 1885. https://doi.org/10.3390/cells11121885.
[6] Fu, X., Cai, H., Quan, S., Ren, Z., Xu, Y., & Jia, L. (2024). Immune cells in Alzheimer’s disease: insights into pathogenesis and potential therapeutic targets. Medical Review, 5(3), 179–202. https://doi.org/10.1515/mr-2024-0064.
[7] Wu, A., & Zhang, J. (2023). Neuroinflammation, memory, and depression: new approaches to hippocampal neurogenesis. Journal of Neuroinflammation, 20(1). https://doi.org/10.1186/s12974-023-02964-x.
[8] Liu, L., Liu, J., Bao, J., Bai, Q., & Wang, G. (2020). Interaction of Microglia and Astrocytes in the Neurovascular Unit. Frontiers in Immunology, 11. https://doi.org/10.3389/fimmu.2020.01024.
[9] Bridlance, C., & Thion, M. S. (2023). Multifaceted microglia during brain development: Models and tools. Frontiers in Neuroscience, 17. https://doi.org/10.3389/fnins.2023.1125729.
[10] Li, L., Cui, X., Zhu, B., Zhou, L., Guo, Y., Liu, T., & Shi, Y. (2025). Role of astrocytes in the pathogenesis of perinatal brain injury. Molecular Medicine, 31(1). https://doi.org/10.1186/s10020-025-01328-w.
[11] Laketa, D., & Lavrnja, I. (2024). Extracellular Purine Metabolism—Potential Target in Multiple Sclerosis. Molecular Neurobiology, 61(10), 8361–8386. https://doi.org/10.1007/s12035-024-04104-9.
[12] Zhu, Q., Song, Y., Qian, Y., Zhang, R., Xue, J., & Hou, Y. (2025). Targeting glial dysfunction in Alzheimer’s disease: insights into pathogenesis and emerging therapeutics. Ageing and Neurodegenerative Diseases, 5(3). https://doi.org/10.20517/and.2025.25.
[13] Mira, R. G., Lira, M., & Cerpa, W. (2021). Traumatic Brain Injury: Mechanisms of Glial Response. Frontiers in Physiology, 12. https://doi.org/10.3389/fphys.2021.740939.
[14] Pasquini, J. M., & Correale, J. D. (2025). The immunological role of oligodendrocytes: beyond myelin maintenance. Discovery Immunology, 4(1). https://doi.org/10.1093/discim/kyaf005.
[15] Neumaier, E. E., Rothhammer, V., & Linnerbauer, M. (2023). The role of midkine in health and disease. Frontiers in Immunology, 14. https://doi.org/10.3389/fimmu.2023.1310094.
[16] Rustenhoven, J., Jansson, D., Smyth, L. C., & Dragunow, M. (2017). Brain Pericytes As Mediators of Neuroinflammation. Trends in Pharmacological Sciences, 38(3), 291–304. https://doi.org/10.1016/j.tips.2016.12.001.
[17] Colombo, E., & Farina, C. (2016). Astrocytes: Key Regulators of Neuroinflammation. Trends in Immunology, 37(9), 608–620. https://doi.org/10.1016/j.it.2016.06.006.
[18] Kwon, H. S., & Koh, S.-H. (2020). Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Translational Neurodegeneration, 9(1). https://doi.org/10.1186/s40035-020-00221-2.
[19] Zhao, Y., Huang, Y., Cao, Y., & Yang, J. (2024). Astrocyte-Mediated Neuroinflammation in Neurological Conditions. Biomolecules, 14(10), 1204. https://doi.org/10.3390/biom14101204.
[20] Patani, R., Hardingham, G. E., & Liddelow, S. A. (2023). Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nature Reviews Neurology, 19(7), 395–409. https://doi.org/10.1038/s41582-023-00822-1.
[21] Ma, D., Jin, S., Li, E., Doi, Y., Parajuli, B., Noda, M., Sonobe, Y., Mizuno, T., & Suzumura, A. (2013). The neurotoxic effect of astrocytes activated with toll-like receptor ligands. Journal of Neuroimmunology, 254(1–2), 10–18. https://doi.org/10.1016/j.jneuroim.2012.08.010.
[22] Brambilla, R. (2019). The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathologica, 137(5), 757–783. https://doi.org/10.1007/s00401-019-01980-7.
[23] Nourbakhsh, F., Read, M. I., Barreto, G. E., & Sahebkar, A. (2021). Astrocytes and Inflammasome: A Possible Crosstalk in Neurological Diseases. Current Medicinal Chemistry, 28(24), 4972–4994. https://doi.org/10.2174/0929867328666210301105422.
[24] Michinaga, S., & Koyama, Y. (2021). Pathophysiological Responses and Roles of Astrocytes in Traumatic Brain Injury. International Journal of Molecular Sciences, 22(12), 6418. https://doi.org/10.3390/ijms22126418.
[25] Nakano-Kobayashi, A., Canela, A., Yoshihara, T., & Hagiwara, M. (2023). Astrocyte-targeting therapy rescues cognitive impairment caused by neuroinflammation via the Nrf2 pathway. Proceedings of the National Academy of Sciences, 120(33). https://doi.org/10.1073/pnas.2303809120.
[26] Lv, M., Duan, Z., Tan, J., Liu, J., Wang, Q., Wang, C., Zhang, Z., Sun, X., Liu, R., & Cui, Y. (2025). PHGDH-mediated serine synthesis in astrocytes supports neuroinflammation by sustaining NADH level to promote histone acetylation. Cell Death & Disease, 16(1). https://doi.org/10.1038/s41419-025-07732-8.
[27] Champagne-Jorgensen, K., & Gommerman, J. (2024). Astrocytes ACLYmate to chronic neuroinflammation. Trends in Immunology, 45(5), 320–321. https://doi.org/10.1016/j.it.2024.04.003.
[28] Colonna, M., & Butovsky, O. (2017). Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annual Review of Immunology, 35(1), 441–468. https://doi.org/10.1146/annurev-immunol-051116-052358.
[29] Kwon, H. S., & Koh, S.-H. (2020). Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Translational Neurodegeneration, 9(1). https://doi.org/10.1186/s40035-020-00221-2.
[30] Ajoolabady, A., Kim, B., Abdulkhaliq, A. A., Ren, J., Bahijri, S., Tuomilehto, J., Borai, A., Khan, J., & Pratico, D. (2025). Dual role of microglia in neuroinflammation and neurodegenerative diseases. Neurobiology of Disease, 216, 107133. https://doi.org/10.1016/j.nbd.2025.107133.
[31] Feldman, R. A. (2022). Microglia orchestrate neuroinflammation. eLife, 11. https://doi.org/10.7554/elife.81890.
[32] Maurya, S. K., Gupta, S., & Mishra, R. (2023). Transcriptional and epigenetic regulation of microglia in maintenance of brain homeostasis and neurodegeneration. Frontiers in Molecular Neuroscience, 15. https://doi.org/10.3389/fnmol.2022.1072046.
[33] Luo, E. Y., & Sugimura, R. R. (2024). Taming microglia: the promise of engineered microglia in treating neurological diseases. Journal of Neuroinflammation, 21(1). https://doi.org/10.1186/s12974-024-03015-9.
[34] Tang, L.-H., & Yao, Y.-B. (2024). Study on the role of peripheral immune cells in cerebral ischemia. New Cell, 1–12. https://doi.org/10.61958/ncql1036.
[35] Zhang, Q., Yang, G., Luo, Y., Jiang, L., Chi, H., & Tian, G. (2024). Neuroinflammation in Alzheimer’s disease: insights from peripheral immune cells. Immunity & Ageing, 21(1). https://doi.org/10.1186/s12979-024-00445-0.
[36] Müller, L., & Di Benedetto, S. (2025). Neuroimmune crosstalk in chronic neuroinflammation: microglial interactions and immune modulation. Frontiers in Cellular Neuroscience, 19. https://doi.org/10.3389/fncel.2025.1575022.
[37] Yang, S., Qin, C., Hu, Z.-W., Zhou, L.-Q., Yu, H.-H., Chen, M., Bosco, D. B., Wang, W., Wu, L.-J., & Tian, D.-S. (2021). Microglia reprogram metabolic profiles for phenotype and function changes in central nervous system. Neurobiology of Disease, 152, 105290. https://doi.org/10.1016/j.nbd.2021.105290.



