Ferroptosis
Ferroptosis is a form of programmed cell death associated with iron-dependent lipid peroxidation, involving various physiological processes such as iron metabolism, lipid metabolism, oxidative stress, and biosynthesis. Studies have shown that ferroptosis is involved in the development and progression of multiple diseases, including inflammatory diseases, cancers, infectious diseases, and systemic disorders.
Macrophages
Macrophages are immune cells with phagocytic functions, widely distributed in various tissues. They secrete cytokines, generate reactive oxygen species (ROS), mediate inflammatory responses, and regulate the metabolism of iron, lipids, and amino acids, playing a crucial role in tissue homeostasis. Under different microenvironmental stimulation, macrophages differentiate into distinct subtypes, most commonly the M1 and M2 phenotypes.
Both ferroptosis and macrophages are current research hotspots. Increasing evidence suggests a close relationship between macrophages and ferroptosis, providing new targets for a deeper understanding of disease mechanisms and therapeutic strategies. This article shares some research on the interaction between ferroptosis and macrophages.
Table of Contents
1. Macrophage Recognition and Clearance of Ferroptotic Cells
2. Macrophage-Mediated Iron Homeostasis and Its Role in Ferroptosis
3. Ferroptosis Regulates Macrophage Polarization and Tumor Immunity
01 Macrophage Recognition and Clearance of Ferroptotic Cells
Macrophages can clear cells undergoing ferroptosis. HMGB1 released by ferroptotic cells interacts with AGER on macrophages, mediating inflammatory responses. Molecules such as CCL2 or CCL7 can initiate macrophage recruitment and chemotaxis to enhance immune responses. Additionally, TLR2 on macrophages recognizes and binds to SAPE-OOH on the surface of ferroptotic cells, enhancing phagocytosis and aiding in the clearance of ferroptotic cells.
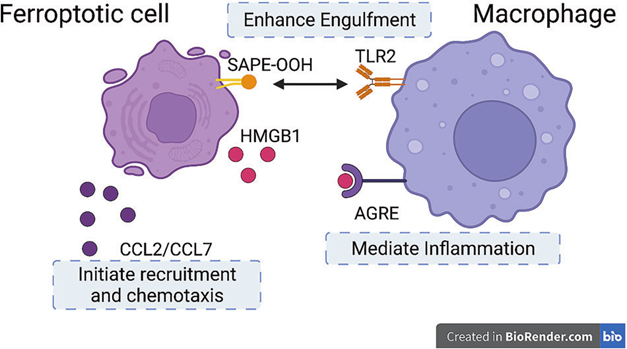
Fig. 1 Recognition Between Macrophages and Ferroptotic Cells[1].
02 Macrophage-Mediated Iron Homeostasis and Its Role in Ferroptosis
Macrophages are central to regulating iron homeostasis, with two main iron sources. First, macrophages generate iron by phagocytosing senescent red blood cells, serving as a primary source of bioavailable iron in the body. Second, extracellular iron (Fe3+) bound to transferrin (TF) can enter macrophages via TFR1. Under normal conditions, iron levels are maintained through the hepcidin/ferroportin regulatory system. When this balance is disrupted, abnormal iron metabolism may lead to excess reactive iron, causing iron deposition and lipid peroxidation, ultimately triggering ferroptosis. Moreover, cytokines produced by macrophages can regulate intracellular LOX activity, thereby inducing ferroptosis.
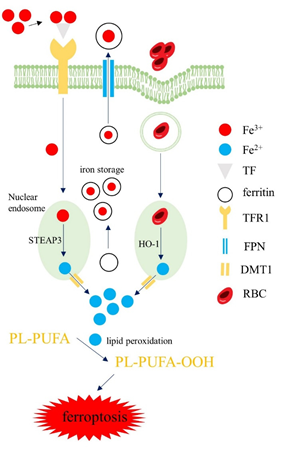
Fig. 2 Iron Homeostasis in Macrophages[2].
03 Ferroptosis Regulates Macrophage Polarization and Tumor Immunity
Ferroptosis promotes macrophage polarization by affecting iron metabolism or other metabolic reprogramming within macrophages. Studies indicate that ferroptosis induces iron overload in macrophages, promoting polarization toward the M1 phenotype, increasing production of inflammatory factors, and impairing tissue repair and immunoregulatory functions. M1 and M2 macrophages not only exhibit different iron metabolic states but also vary in their susceptibility to ferroptosis. M1 macrophages are less sensitive to ferroptosis induced by the GPX4 inhibitor RSL3 due to elevated levels of nitric oxide radicals, which inhibit lipid peroxidation. In contrast, M2 macrophages express less iNOS and are more sensitive to RSL3-induced ferroptosis. Recent research aims to treat tumors by altering macrophage polarization, disrupting the tumor microenvironment, and inducing ferroptosis in tumor cells.
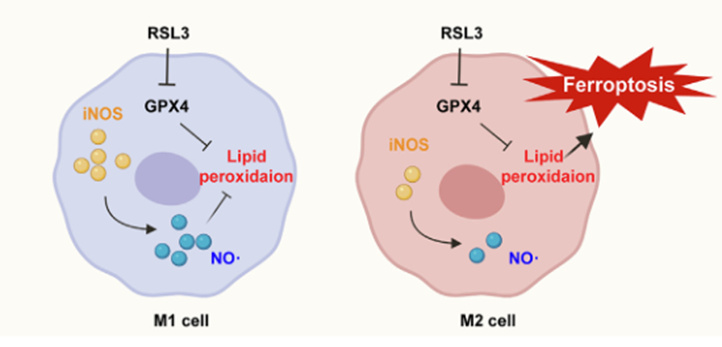
Fig. 3 Ferroptosis in M1 and M2 Macrophages[3].
Other studies have found that ferroptosis, mediated by iron transport into bacterial vesicles via ferroportin, can trigger ferroptosis in intracellular bacteria, aiding macrophages in combating such infections. This antibacterial effect effectively suppresses intracellular bacteria without harming macrophages. In antitumor therapy, targeting xCT-mediated ferroptosis and pro-tumor polarization in macrophages has been shown to effectively combat hepatocellular carcinoma (HCC) and enhance the efficacy of anti-PD-1/ PD-L1 responses.
In summary, current research indicates a significant connection between macrophages and ferroptosis. Although the precise regulatory mechanisms of their interaction are not fully understood and require further investigation, elucidating the interplay between ferroptosis and macrophages is crucial for developing innovative therapeutic strategies and evaluating their efficacy, particularly in diseases such as cancer.
References:
[1] Yang Y , Wang Y , Guo L ,et al. Interaction between macrophages and ferroptosis[J]. Cell Death & Disease, 2022, Apr 16;13(4):355.
[2] Ma J, Zhang H, Chen Y, et al. The Role of Macrophage Iron Overload and Ferroptosis in Atherosclerosis[J]. Biomolecules, 2022, 12(11): 1702.
[3] Xu S, Min J, Wang F. Ferroptosis: an emerging player in immune cells[J]. Science Bulletin, 2021, 66(22): 2257-60.



