Mitochondria is universally acknowledged as the cell’s "powerhouses" by virtue of their central role in aerobic oxidative phosphorylation, these organelles execute a multitude of essential biological processes beyond adenosine triphosphate (ATP) biosynthesis. As critical signaling hubs, mitochondria engage in bidirectional communication with the nucleus and other sub-cellular compartments to sustain cellular homeostasis, facilitate adaptive responses to diverse stress stimuli, and regulate cell fate determination during development. Accordingly, mitochondria have become a preeminent focus of investigations into both physiological and pathological processes, encompassing normal tissue homeostasis, metabolic regulation, neurodegeneration, immune modulation, and infectious diseases[1].
Over the past five decades, an extensive body of research has been dedicated to the exploration of mitochondrial metabolism, employing a broad spectrum of sophisticated experimental methodologies[2]. This article reviews the assessment of mitochondrial function assays using fluorescent probes, contrasts this approach with conventional techniques, and illustrates its application with specific examples.
Table of Contents
1. Mitochondrial function assays
2. The principle of fluorescence-based mitochondrial assays
3. How to perform ATP production assays by using fluorescence-based assay
4. How to perform reactive oxygen species (ROS) assays by using fluorescence-based assay
5. The advantages of fluorescence-based mitochondrial assays
6. Applications of fluorescence-based mitochondrial assays in drug discovery
01 Mitochondrial function assays
Mitochondria is semi-autonomous organelles present in the majority of eukaryotic cells, characterized by a bilayered membrane structure comprising an outer membrane, an intermembrane space, and an inner membrane. These organelles play pivotal roles in a diverse array of cellular processes, including cellular metabolism (eg. the biosynthesis of ATP to sustain cellular energy demands), signal transduction (eg. the regulation of intracellular calcium ion homeostasis), and the regulation of cell death (eg. the release of cytochrome c). Dysregulation of mitochondrial structure and function can trigger a cascade of intracellular signaling events, induce oxidative stress, and initiate programmed cell death (apoptosis), thereby contributing to the onset and progression of a broad spectrum of human diseases[3]. Thus, the accurate detection of mitochondrial abnormalities is of paramount importance, and a variety of specialized mitochondrial assays have been developed for this purpose[3].
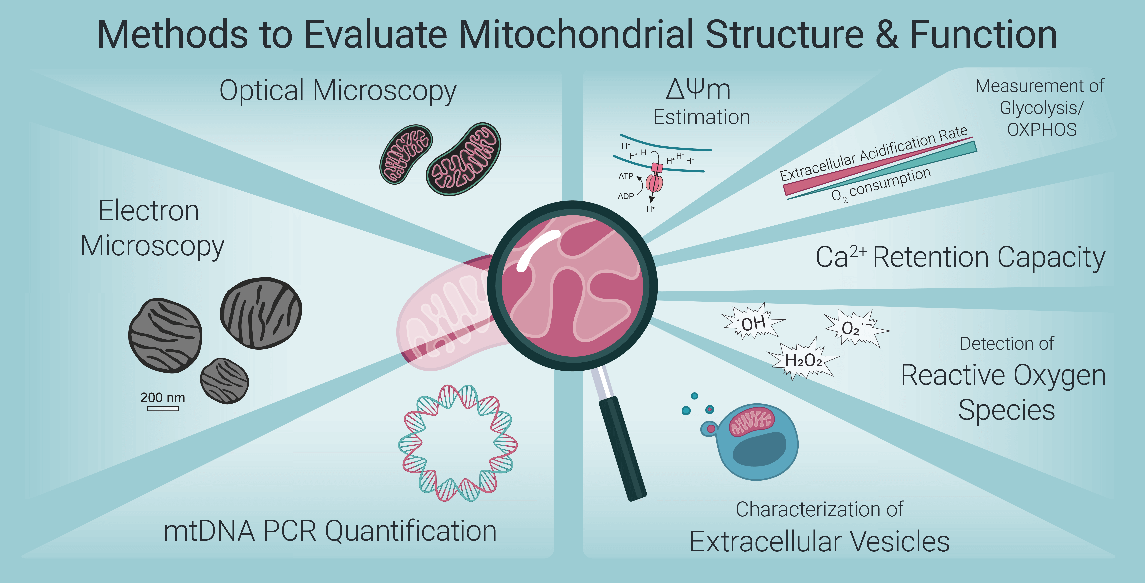
Fig. 1 Methods to Evaluate Mitochondrial Structure & Function[4].
Recent advances in fluorescent imaging technologies have substantially enhanced our capacity to analyze mitochondrial morphology and dynamics, as well as to precisely quantify levels of specific metabolites and ions within its subcompartments, including the mitochondrial membranes and matrix[5]. A diverse array of fluorescent probes and potentiometric dyes has been increasingly employed for the quantitative assessment of global mitochondrial content, membrane potential, oxidative stress, apoptosis, and intracellular calcium ion (Ca²⁺) concentrations[6-9].
02 The principle of fluorescence-based mitochondrial assays
A multitude of indicators are available for the assessment of mitochondrial function. Below, we briefly overview fluorescence-based detection methodologies, with a focus on one representative assay per indicator.
The ATP production rate serves as a key indicator of oxidative phosphorylation activity in isolated mitochondria. Specifically, the ATP generated is quantified through a bioluminescent reaction catalyzed by firefly luciferase: luciferin reacts with ATP to yield oxyluciferin, adenosine monophosphate (AMP), and photons, with the emitted light detected using a luminometer[3].
For intracellular ROS measurement, the fluorescent probe 2',7'-dichlorodihydrofluorescein diacetate (DCHF-DA) is widely utilized owing to its distinctive physicochemical characteristics. This probe is inherently non-fluorescent and possesses the ability to freely permeate the cell membrane. Upon entry into cells, DCHF-DA undergoes hydrolysis catalyzed by intracellular esterases, yielding 2',7'-dichlorodihydrofluorescein (DCHF): a membrane-impermeable metabolite that remains trapped within the cellular compartment. This intracellular retention enables specific and stable labeling of target cells. In the presence of intracellular ROS, DCHF is rapidly oxidized to form the highly fluorescent compound 2',7'-dichlorofluorescein (DCF). Notably, the fluorescence intensity of DCF is directly proportional to the intracellular ROS concentration, allowing quantitative assessment of ROS levels[10].
Detection of mitochondrial permeability transition pores (mPTP) assay
The calcein-cobalt fluorescent probe assay has emerged as a promising method for detecting mitochondrial permeability transition pore (mPTP) opening, featuring simplicity of operation and high sensitivity. Acetylmethoxymethyl ester-conjugated calcein(Calcein-AM) is utilized for fluorescent labeling of live cells, as the AM moiety enhances the probe’s hydrophobicity to facilitate penetration across the plasma membrane of viable cells. Upon intracellular entry, calcein-AM is hydrolyzed by endogenous esterases, releasing calcein, a highly fluorescent and polar metabolite. When cells are co-incubated with calcein and cobalt ions (Co²⁺), both molecules diffuse into the cytoplasm; notably, calcein is selectively sequestered by mitochondria. Mitochondria-localized calcein emits strong fluorescence, whereas calcein remaining in the cytoplasm or released from mitochondria into the cytoplasmic compartment is rapidly quenched by Co²⁺. Under physiological conditions, mPTP opens transiently, and any calcein leaking from mitochondria into the cytoplasm is immediately quenched by cytoplasmic Co²⁺, maintaining stable mitochondrial fluorescence. In contrast, under pathological conditions such as calcium overload or oxidative stress, mPTP undergoes sustained opening. This prolonged pore opening permits cytoplasmic Co²⁺ to enter mitochondria, where it quenches intramitochondrial calcein fluorescence. Consequently, a progressive reduction in mitochondrial fluorescence intensity is observed, which directly reflects the extent of mPTP opening[10].
Mitochondrial membrane potential(ΔΨm) assay
The membrane potential across the mitochondrial inner membrane serves as a pivotal parameter for assessing global mitochondrial function, as it reflects the dynamic balance between the reduction potential of proton pumps within the electron transport chain (ETC) and the rate of ion conductance: specifically, the re-entry of protons (H⁺) across the inner membrane into the mitochondrial matrix[3].JC-1 exists in two distinct forms(monomer and polymer)with differing emission spectra. In normal cells, the mitochondrial membrane potential is relatively high, prompting JC-1 to localize in the mitochondrial matrix as polymers that emit red fluorescence. In the early stages of apoptosis, a reduction in mitochondrial membrane potential induces JC-1 to exist as monomers, which also localize in the mitochondrial matrix and emit green fluorescence.
Oxygen consumption rate (OCR) assay
Mitochondrial oxidative phosphorylation in cells consumes oxygen to synthesize ATP, which serves as the primary energy source to support cellular growth and metabolic activities. Consequently, OCR is recognized as a key readout for evaluating mitochondrial functional capacity. The detection of OCR relies on a phosphorescent probe with high oxygen sensitivity. In a sealed assay system, as cells consume oxygen, the reduction in dissolved oxygen concentration leads to a proportional increase in the probe’s phosphorescence intensity. OCR is then quantitatively determined by monitoring the dynamic changes in phosphorescence values over time. For comprehensive functional analysis, OCR measurements are typically combined with complementary assays, including the carboxyfluorescein succinimidyl ester (CFSE) cell proliferation assay and the 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) glucose uptake assay.
03 How to perform ATP production assays by using fluorescence-based assay
The second section provides a brief overview of various fluorescence-based detection methodologies. In this and subsequent sections, specific detection approaches will be elaborated to facilitate a comprehensive understanding of fluorescent-based mitochondrial function assessment, using ATP detection kit (via chemiluminescence, a specialized fluorescence-related technique) and ROS fluorescence detection as representative examples.
For ATP assay, ATP measurement kit are generally used, the experimental procedure is as follows:
First, prepare a working solution containing luciferase, luciferin sodium, surfactant, and activator. Generate a standard curve using ATP solutions of known concentrations (e.g., 1-100 μM). Centrifuge cell samples to remove culture medium and resuspend pellets in phosphate-buffered saline (PBS, 0.01 M, pH 7.4). Add standards or cell suspensions to microplate wells, followed immediately by equal volumes of working solution. After brief incubation at room temperature, measure chemiluminescence intensity using a microplate reader. Calculate intracellular ATP content by comparing sample readings to the standard curve.
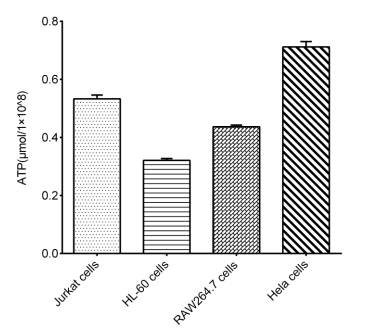
Fig. 2 Detection Results of ATP in Multiple types of cells.
04 How to perform reactive oxygen species (ROS) assays by using fluorescence-based assay
The fluorescent probe-based method for reactive oxygen species assay relies on the use of specific probes that undergo selective reaction with intracellular ROS, resulting in the generation of detectable fluorescence signals. These fluorescence signals can then be visualized and quantitatively analyzed using specialized equipment such as fluorescence microscopes and flow cytometers. Among the commonly used probes, DCFH-DA is the most widely employed, as it emits green fluorescence upon oxidation by ROS. The detailed experimental procedure for ROS detection using DCFH-DA is as follows:
First, add the DCFH-DA probe to the cell culture system. The optimal concentration of DCFH-DA varies depending on the cell density and specific cell type, and should be determined empirically for each experimental setup.
Second, incubate the cells at 37°C in a humidified 5% CO₂ incubator for a period ranging from 30 minutes to several hours; typically, an incubation time of 30-60 minutes is sufficient for most cell types. It is important to note that the exact incubation duration is influenced by factors including cell type, experimental stimulation conditions, and the final concentration of DCFH-DA used.
Finally, following incubation, centrifuge the cell samples to pellet the cells, and wash the pellets with an appropriate buffer (e.g., phosphate-buffered saline) to remove unincorporated probe. The intracellular ROS levels can then be evaluated by detecting the fluorescence intensity using a fluorescence microplate reader, or by capturing fluorescence images with a fluorescence microscope, as required by the experimental design.
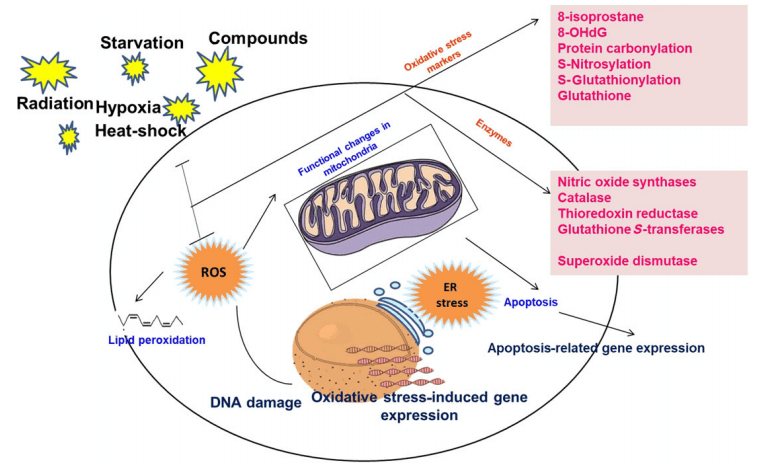
Fig. 3 ROS-Mediated Events and Detection Markers[11].
05 The advantages of fluorescence-based mitochondrial assays
Fluorescence-based methodologies offer several distinct advantages for assessing mitochondrial function:
Enhanced Sensitivity: Fluorometric assays typically offer 2-4 orders of magnitude greater sensitivity than colorimetric methods, enabling detection of minute quantities of analytes in small sample volumes.
Versatile Detection Platforms: Fluorescence signals can be detected using various instruments including microscopes, flow cytometers, and microplate readers, providing results in highly intuitive formats suitable for both imaging and quantitative analysis.
Live-Cell Compatibility: Most fluorescent probes enable direct detection in living cells without complex processing, allowing real-time monitoring of dynamic mitochondrial processes.
Multiplexing Capability: Fluorescence-based assays can be combined with other analytical methods through use of probes with distinct excitation and emission spectra, enabling simultaneous assessment of multiple parameters.
While colorimetric assays offer advantages in cost and direct enzyme activity measurement, fluorescence-based methods provide superior sensitivity and flexibility for functional assessment in intact cells.
06 Applications of fluorescence-based mitochondrial assays in drug discovery
Mitochondrial function studies are pivotal for elucidating disease mechanisms and discovering novel therapeutic targets. Consequently, a variety of drug screening strategies have been established based on the assessment of mitochondrial function.
One prominent screening approach focuses on mPTP in human neurons. This strategy combines evaluations of ΔΨm and mPTP activity. The process typically begins with the JC-1 assay to screen candidate drugs for their impact on ΔΨm. Compounds that show potential are then further validated using probes such as Calcium Yellow Green AM to characterize their effects on mPTP opening[12]. This sequential screening method efficiently identifies lead compounds for development.
Another well-established strategy assesses mitochondrial respiratory function by measuring intracellular ATP levels or utilizing oxygen-sensitive fluorescent probes. In a sealed assay system, cellular oxygen consumption lowers dissolved oxygen concentration. This reduction leads to a proportional increase in the phosphorescence intensity of the probe. The oxygen consumption rate (OCR) is quantified in real time by monitoring this fluorescent signal over time. Candidate drugs that induce significant alterations in OCR are selected for further validation.
Fluorescence-based methods are recognized as straightforward, intuitive, and efficient for identifying compounds that modulate mitochondrial function. Their application in preclinical drug safety assessment and toxicity screening is increasingly important.
In summary, the rational development of drug screening methodologies relies on a comprehensive understanding of mitochondrial function and corresponding detection technologies. Among the available approaches, fluorescent probe-based methods stand out for their directness, intuitiveness, and efficiency in identifying candidate compounds that target mitochondrial function.
Elabscience® Quick Overview of Popular Products
Table 1. Fluorometric Assay Kits for Mitochondrial Functional Research
|
Cat. No. |
Product Name |
|
E-BC-F064 |
Mitochondrial Permeability Transition Pore (mPTP) Fluorometric Assay Kit |
|
E-BC-F070 |
Enhanced Oxygen Consumption Rate (OCR) Fluorometric Assay Kit |
|
E-BC-F300 |
ATP Assay Kit |
|
E-CK-A301 |
Mitochondrial Membrane Potential Assay Kit (with JC-1) |
|
E-CK-A345 |
CFSE proliferation Assay Kit |
|
E-CK-A441 |
2-nbdg glucose uptake assay Kit |
|
E-BC-K138-F |
Reactive Oxygen Species (ROS) Fluorometric Assay Kit (Green) |
|
E-CK-A401 |
MitoBright Green Probe Assay Kit |
|
E-CK-A402 |
MitoBright Red Probe Assay Kit |
|
E-CK-A403 |
MitoBright Deep Red Probe Assay Kit |
|
E-BC-F100 |
Fluo-4 Calcium Fluorometric Assay Kit |
|
E-BC-F008 |
Mitochondrial Superoxide Fluorometric Assay Kit |
|
E-BC-F005 |
Reactive Oxygen Species (ROS) Fluorometric Assay Kit (Red) |
References:
[1] Rossmann, M. P., Dubois, S. M., Agarwal, S., & Zon, L. I. (2021). Mitochondrial function in development and disease. Disease models & mechanisms, 14(6), dmm048912.
[2] Zhang, Y., & Avalos, J. L. (2017). Traditional and novel tools to probe the mitochondrial metabolism in health and disease. Wiley Interdisciplinary Reviews: Systems Biology and Medicine, 9(2), e1373.
[3] Yin, Y., & Shen, H. (2022). Common methods in mitochondrial research. International Journal of Molecular Medicine, 50(4), 126
[4] Rickard, B. P., Overchuk, M., Chappell, V. A., Kemal Ruhi, M., Sinawang, P. D., Nguyen Hoang, T. T., Akin, D., Demirci, U., Franco, W., Fenton, S. E., Santos, J. H., & Rizvi, I. (2023). Methods to Evaluate Changes in Mitochondrial Structure and Function in Cancer. Cancers, 15(9), 2564. https://doi.org/10.3390/cancers15092564.
[5] Marín-García, J. (2012). Methods to study mitochondrial structure and function. In Mitochondria and their role in cardiovascular disease (pp. 13-27). Boston, MA: Springer US.
[6] Mathur A, Hong Y, Kemp BK, Barrientos AA, Erusalimsky JD. Evaluation of fl uorescent dyes for the detection of mitochondrial membrane potential changes in cultured cardiomyocytes. Cardiovasc Res. 2000;46(1):126–38.
[7] Dykens JA, Stout AK. Assessment of mitochondrial membrane potential in situ using single potentiometric dyes and a novel fluorescence resonance energy transfer technique. Methods Cell Biol. 2001;65:285–309.
[8] Haugland RP. Handbook of fl uorescent probes and research products. 9th ed. Eugene, OR: Molecular Probes, Inc; 2002.
[9] Duchen MR, Surin A, Jacobson J. Imaging mitochondrial function in intact cells. Methods Enzymol. 2003;361:353–89.
[10] Perry, C. G., Kane, D. A., Lanza, I. R., & Neufer, P. D. (2013). Methods for assessing mitochondrial function in diabetes. Diabetes, 62(4), 1041-1053.
[11]Ediriweera, M. K., Tennekoon, K. H., & Samarakoon, S. R. (2019). In vitro assays and techniques utilized in anticancer drug discovery. Journal of Applied Toxicology, 39(1), 38-71
[12] Woollacott, A. J., & Simpson, P. B. (2001). High throughput fluorescence assays for the measurement of mitochondrial activity in intact human neuroblastoma cells.SLAS Discovery,6(6), 413-420.



