Mitochondria play dual pivotal roles in the crosstalk between cancer and metabolism, sustaining basal energy production. It is meaningful to determine whether mitochondria function properly but it is also challenging. In this issue, we present a comprehensive overview and troubleshooting guide for mitochondrial function assays, with a specific focus on strategies to overcome weak mitochondria detection. We highlight critical factors affecting assay reliability, such as sample integrity, assay condition control, and instrumentation calibration, and detail optimized protocols for core assays. These solutions address common problems to enhance the sensitivity, accuracy, and reproducibility of mitochondrial functional assessments, thereby supporting cancer and metabolism research.
Table of Contents
1. Factors affecting mitochondrial function assay reliability
2. How to optimize mitochondrial function assay protocols
3. Solutions for weak signal in mitochondrial assays
4. Common problems in mitochondrial respiration assays
5. Troubleshooting mitochondrial membrane potential assays
6. How to fix inconsistent results in ATP production assays
01 Factors affecting mitochondrial function assay reliability
Mitochondrial function assays are indispensable tools for investigating cellular bioenergetics and metabolic homeostasis. The validity of results hinges on stringent control of technical and biological variables. Systematic errors at any step can introduce bias or irreproducibility, necessitating focus on three pivotal aspects: sample integrity, assay condition optimization, and instrumentation & data standardization.
Sample Integrity: High sample purity is critical. For cell sample processing, using cell separation technology, such as FACS or MACS, can enhance purity. For tissue sample processing, snap-freezing in liquid nitrogen and gentle homogenization in a suitable mitochondrial assay solution help preserve mitochondrial structure and activity. Fresh samples are preferred for optimal results in mitochondria activity assays[1].
Assay Condition Control: Temperature and pH fluctuations can alter enzyme kinetics and metabolic flux. Thus, calibrated equipment and specilized buffered systems (e.g., HEPES) are essential[2]. Besides, reagent stability is vital because some light-sensitive dyes (e.g., JC-1) and substrates degrade under improper storage, leading to signal loss[3]. Aliquoting, vendor verification, and regular quality checks are recommended.
Instrumentation & Data Analysis: Precision depends on calibrated equipment (e.g., OCR sensors, microplate readers) and standardized software protocols. Personnel training ensures consistent operation, avoiding artifacts from sampling or normalization errors.
02 How to optimize mitochondrial function assay protocols
Enhancing data reliability requires multi-dimensional optimization: methodology selection, parameter validation, and rigorous experimental design.
Methodology Selection: Choose techniques based on research goals and sample properties. Oxygen consumption rate (OCR) and adenosine triphosphate (ATP) assays are suitable for analyzing energetic status (e.g., in tumor cells)[4], while mitochondrial membrane potential (ΔΨm, detected by JC-1) or reactive oxygen species (ROS) detection targets pathologies involving depolarization or oxidative stress. Adherent cells favor microscopy and seahorse assay, suspension cells favor flow cytometry.
Reagent & Parameter Validation: Commercial kits require model-specific validation using positive and negative controls because the number, integrity and function of mitochondria may change. Titrate probe and substrate concentrations to match different mitochondrial content (e.g., adjust substrates for metabolically impaired hepatocytes)[5].
Experimental Design: Include pharmacological (oligomycin, FCCP) and background controls. ≥3 biological replicates are mandatory; primary tissues require more replicates. Time points must capture kinetic changes within the detection system’s linear range.
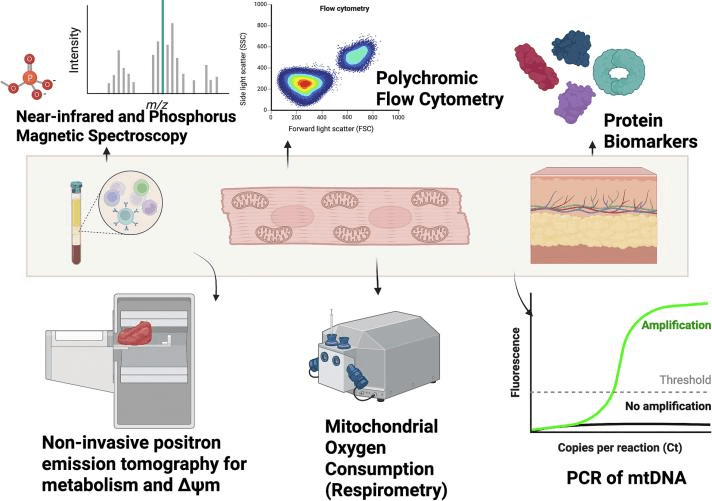
Fig. 1 Mitochondrial function can be measured by a variety of experimental techniques[6].
03 Solutions for weak signal in mitochondrial assays
Weak fluorescence or chemiluminescence signals are common issues in mitochondrial assays, often stemming from low staining efficiency, probe degradation, or improper instrument configuration. Systematic troubleshooting and optimization are required.
Instrument Configuration: Verify that the microscope filter set or the flow cytometer's lasers and detection channels are correctly matched to the excitation/emission spectra of the probe used. For example, a dye with excitation/emission wavelengths of 488/530 nm should be used with a FITC filter set; otherwise, signal collection efficiency will be compromised[5]. Furthermore, regularly calibrate flow cytometer lasers and detectors using standard fluorescent beads to ensure optimal performance. For microscopy, inspect and adjust the light source intensity and filter positions to maximize signal collection.
Probe Integrity: Prepare fresh working solutions from aliquoted stock solutions to avoid hydrolysis caused by repeated freeze-thaw cycles. Light-sensitive probes (e.g., JC-1) must be handled under light-protected conditions throughout the procedure to prevent photobleaching.
Staining Parameters: Optimize cell density and dye concentration within the non-toxic range to avoid non-specific binding or dye aggregation. After staining, perform thorough but gentle washes to reduce background fluorescence while retaining specific signals.
Mitochondrial Health: Weak signals may reflect inherently compromised mitochondrial function. Depolarized or structurally damaged mitochondria exhibit reduced probe uptake and retention. It is advisable to include a positive control to verify the dynamic range of the detection system, ensuring the experimental conditions possess adequate sensitivity.
04 Common problems in mitochondrial respiration assays
Mitochondrial respiration assays evaluate cellular bioenergetic function by measuring key parameters such as the oxygen consumption rate (OCR), respiratory chain complex activity, and proton leak. Among these, OCR serves as the most critical indicator. Commonly used detection methods include assays based on the Seahorse analyzer and fluorescent microplate readers. Herein, we focus on fluorescent microplate reader-based OCR assays, addressing common issues and optimization strategies.
Table1. Differences Between the Seahorse and Fluorescent Plate Readers for OCR Measurement
|
Aspect |
Seahorse Analyzer |
Fluorescent Plate Reader |
|
Principle |
Solid-state sensors for real-time O₂/pH[7] |
Fluorescent probes |
|
Operation |
Automated reagent injection |
Manual multi-step interventions |
|
Data Output |
Kinetic curves (dynamic response) |
Endpoint fluorescence intensity |
|
Suitability |
High-resolution dynamics |
High-throughput screening |
Key Considerations for Fluorescent Microplate Reader-Based OCR Assays
Instrument Requirements: The microplate reader must possess fluorescence intensity detection, precise temperature control (typically 37°C), kinetic (time-course) measurement mode, and bottom-read capability (for adherent cells).
Typical Measurement Parameters (Example): Enable kinetic mode and bottom read. Maintain temperature at 37°C. Set excitation/emission wavelengths per probe specifications (e.g., Ex 405 nm, Em 675 nm). Set reading intervals between 1.5-3 minutes, with a total duration of at least 90 minutes to ensure sufficient data points for curve construction.
Substrate Optimization: Use fresh pyruvate and malate (complex I) or succinate (complex II) solutions. Permeabilization aids substrate access. Titrate concentrations to avoid underestimation.
Inhibitor Considerations: Validate specificity (e.g., rotenone vs. flavoproteins). Combine inhibitors to confirm complex isolation.
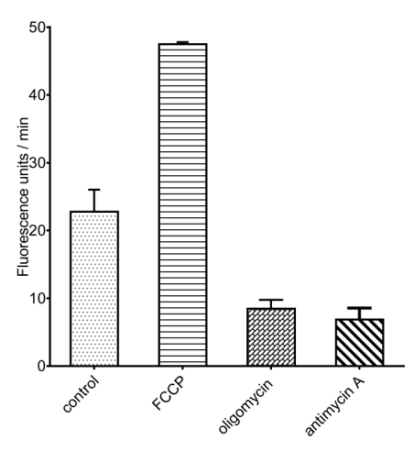
Fig. 2 A549 cells are treated with mitochondrial respiratory chain inhibitors (oligomycin, antimycin A) or uncoupling agents (FCCP), followed by monitoring changes of OCR.
05 Troubleshooting mitochondrial membrane potential assays
JC-1is widely used to detect ΔΨm changes, a key marker of mitochondrial health and early apoptosis. This note addresses common experimental challenges and provides optimized protocols for reliable, reproducible data.
5.1 Red crystalline aggregates in working solution.
Cause: Poor aqueous solubility; reversed dilution sequence (buffer added before water dilution).
Optimized Protocol: thaw and briefly centrifuge components prior to use.Dilute JC-1 stock in ultrapure water per manufacturer instructions, vortex gently.Add concentrated assay buffer to reach 1X working concentration.
For slight precipitation: Incubate at 37°C for 5-10 minutes with periodic vortexing. Avoid prolonged sonication (dye degradation risk).
5.2 Can JC-1 be used to assess mitochondrial membrane potential in tissue samples?
The JC-1 assay can be applied to tissue samples; however, the tissue must first be processed into a single-cell suspension. Following preparation, the procedure for suspension cells should be followed. It is important to note that the enzymatic and mechanical dissociation steps during tissue processing may induce cellular stress, potentially leading to false-positive results. Therefore, optimization of the dissociation protocol is essential. As an alternative approach, mitochondria can be isolated directly from the tissue, followed by incubation with JC-1 for mitochondrial membrane potential assessment.
5.3 Is it feasible to stain adherent cells with JC-1 prior to trypsinization for subsequent flow cytometry analysis?
Harvesting cells to obtain a single-cell suspension prior to staining is recommended, as in situ staining followed by trypsinization can result in uneven dye loading due to variations in cell density and contact inhibition.
5.4 Is the JC-1 Assay Compatible with Paraffin-Embedded or Frozen Tissue Sections?
Incompatible: The JC-1 is a live-cell kinetic assay. Fixation (formaldehyde or methanol) permeabilizes membranes, abolishing ΔΨm and dissociating J-aggregates. Fixed cells or tissue sections are unsuitable.
Successful JC-1 assay implementation requires attention to dye preparation, sample-specific protocols (adherent and suspension cells or tissue), and timely analysis. Use appropriate controls (CCCP or FCCP for depolarization) to ensure fluorescence ratios accurately reflect physiological ΔΨm.
06 How to fix inconsistent results in ATP production assays
ATP is a fundamental indicator of mitochondrial function and cellular viability, routinely quantified by bioluminescence assays in drug discovery and microbiological testing. This protocol details a optimized procedure to enhance lysis efficiency, mitigate matrix interference, and stabilize reaction kinetics, thereby ensuring reliable assessment of metabolic activity.
6.1 Optimized Lysis and ATP Stabilization
Inconsistent cell lysis represents a major source of experimental variability in ATP quantification assays to achieve uniform and complete lysis, it is critical to employ a suitable lysis buffer, such as that provided in commercial ATP measurement kit, which is typically formulated with ATPase-inactivating agents. Ensuring the lysis reagent thoroughly covers the biological sample is essential. Consistent and vigorous mechanical disruption (e.g., via repetitive pipetting or vortexing) must be applied uniformly across all samples. The entire lysis procedure should be conducted on ice to maximally suppress residual ATPase activity. For delayed analysis, lysates should be immediately aliquoted and flash-frozen at -80°C, with repeated freeze-thaw cycles strictly avoided to prevent ATP degradation. Supplementing the lysis buffer with metal chelators, such as EDTA, further enhances ATP stabilization by inhibiting metal-dependent hydrolysis, a feature often incorporated into dedicated ATP detection kit.
6.2 Normalization and Detection of Assay Interference
Raw chemiluminescence signals must be normalized to total protein content or cell number to account for loading differences. To investigate potential interference from the sample matrix or test compounds (e.g., luciferase inhibition, light absorption, or quenching effects), perform an internal standard (spike-in) recovery experiment: add a known concentration of ATP standard to both sample lysates and a blank buffer control, and compare the signal recovery. Significantly lower recovery in sample groups indicates interference, which should be corrected by sample dilution or implementing an ATP extraction and purification step.
6.3 Assay Linearity and Reaction Kinetics
To ensure detection operates within the linear response range, pre-determine the optimal lysate concentration via a dilution series. The luciferase reaction produces a transient luminescent signal that peaks rapidly and then decays. Therefore, the time interval between reagent addition and instrument reading must be strictly controlled.
Recommended Elabscience® Mitochondrial Function Assay Kits
Table 2. Assay Kits for Mitochondrial Functional Research
|
Cat. No. |
Product Name |
|
E-BC-F064 |
Mitochondrial Permeability Transition Pore (mPTP) Fluorometric Assay Kit |
|
E-BC-F070 |
Enhanced Oxygen Consumption Rate (OCR) Fluorometric Assay Kit |
|
E-BC-F300 |
ATP Assay Kit |
|
E-BC-K774-M |
ATP Colorimetric Assay Kit (Enzyme Method) |
|
E-BC-F201 |
Enhanced ATP Chemiluminescence Assay Kit |
|
E-CK-A301 |
Mitochondrial Membrane Potential Assay Kit (with JC-1) |
|
E-BC-F078 |
Mitochondrial Stress Fluorometric Assay Kit |
|
E-BC-K784-M |
Fatty Acid Oxidation (FAO) Colorimetric Assay Kit |
|
E-BC-K138-F |
Reactive Oxygen Species (ROS) Fluorometric Assay Kit (Green) |
|
E-BC-F008 |
Mitochondrial Superoxide Fluorometric Assay Kit |
|
E-BC-F005 |
Reactive Oxygen Species (ROS) Fluorometric Assay Kit (Red) |
References:
[1] Meijer Y, Tröger F, Van den Berg E, et al. POS0932 Systemic upregulated inflammatory cytokines TNFα, IL-6 and IFN-γ in established rheumatoid arthritis patients affect muscle mitochondrial respiration [J]. Annals of the Rheumatic Diseases, 2025, 84: 1057–1058.
[2] Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nature protocols. 2008;3(6):965-976. doi:10.1038/nprot.2008.61
[3] Holzer A-K, Dürr M, Multrus S, et al. PeriTox-M, a Cell-Based Assay for Peripheral Neurotoxicity with Improved Sensitivity to Mitochondrial Inhibitors [J]. Cells, 2025, 14(23): 1929.
[4] Wells M, Brandt L, Beyer, Ph.D A M. The role of telomerase reverse transcriptase in preserving mitochondrial function following doxorubicin Treatment [J]. Physiology, 2025, 40(S1).
[5] Holzer A-K, Dürr M, Multrus S, et al. PeriTox-M, a Cell-Based Assay for Peripheral Neurotoxicity with Improved Sensitivity to Mitochondrial Inhibitors [J]. Cells, 2025, 14(23): 1929.
[6] Sharma E, Fotooh Abadi L, Kombe Kombe JA, et al. Overview of methods that determine mitochondrial function in human disease. Metabolism. 2025;170:156300. doi:10.1016/j.metabol.2025.156300
[7] Plitzko B, Loesgen S. Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in Culture Cells for Assessment of the Energy Metabolism. Bio Protoc. 2018;8(10):e2850. Published 2018 May 20. doi:10.21769/BioProtoc.2850



