In fields like cell biology, disease mechanism research, and drug development, accurate detection of Reactive Oxygen Species (ROS) is crucial for uncovering mechanisms related to oxidative stress. Capturing the dynamic changes of ROS precisely has become a key step in scientific research and drug screening.
This article will break down the core methods and applicable scenarios for ROS detection, focusing on common issues with the popular probe DCFH-DA, to help you quickly establish a reliable detection system.
Table of Contents
1. Applications and Methods for ROS Detection
2. How to Choose a ROS Detection Method?
3. Choosing a ROS Detection Protocol
4. Matching Instruments to Your Goals
5. Key Questions on DCFH-DA Detection
01 Applications and Methods for ROS Detection
Reactive Oxygen Species (ROS), including superoxide anion (·O2-), hydrogen peroxide (H2O2), hydroxyl radical (·OH), and singlet oxygen (1O2), function as a cellular "double-edged sword". At low levels, they regulate key processes like proliferation and differentiation. However, high concentrations trigger oxidative stress, causing damage to nucleic acids, proteins, and lipids, which is linked to diseases such as atherosclerosis, cancer, and neurodegenerative disorders.
Reactive Oxygen Species (ROS), including superoxide (·O2-), H2O2, hydroxyl radical (·OH), and singlet oxygen (1O2), act as a "double‑edged sword" in cells: low levels mediate physiological signaling, while high levels cause oxidative stress and cellular damage, contributing to diseases such as atherosclerosis, cancer, and neurodegeneration.
Main Applications of ROS Detection:
1) Quantifying oxidative stress levels in cells or samples to assess antioxidant system function.
2) Unveiling the role of oxidative stress in disease development and progression.
3) Providing key metrics for drug screening, toxicity evaluation, and antioxidant development.
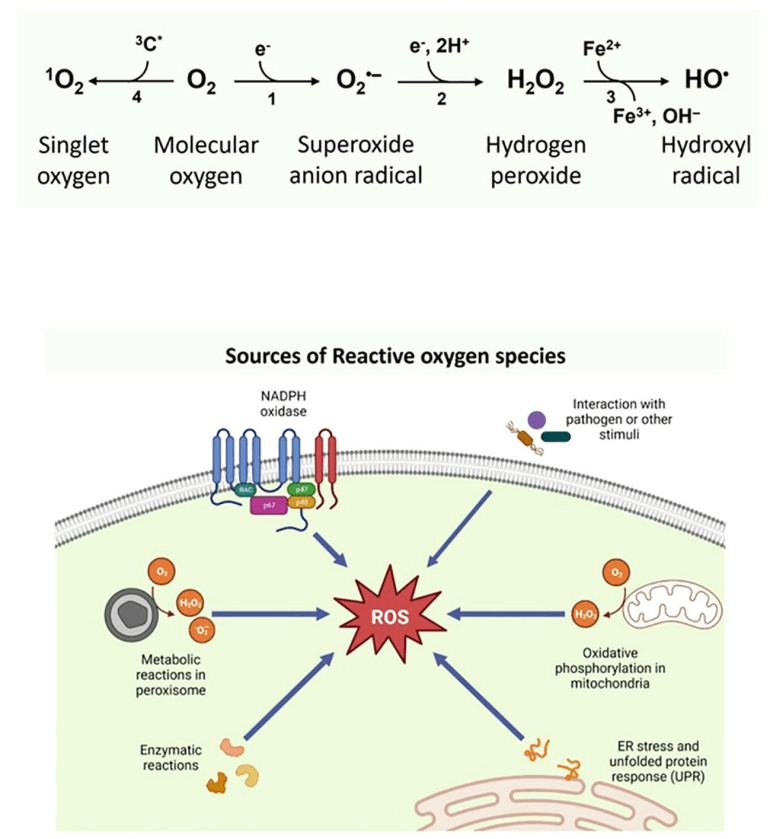
Fig. 1 Types of ROS and their intracellular sources[1].
02 How to Choose a ROS Detection Method?
Current common ROS detection methods include Fluorometric Assays and Electron Paramagnetic Resonance (EPR), which differ in application scenarios and detection targets, as compared below:
Table 1. Comparison of Detection Methods for Reactive Oxygen Species (ROS)
|
Detection Method |
Detection Target |
Sensitivity |
Application Scenario |
Key Technical Points |
|
Fluorometric Assay |
Specific ROS or Total ROS |
High |
Live-cell real-time monitoring, subcellular localization |
Requires optimization of probe concentration & incubation time |
|
EPR |
Specific free radical identification |
Extremely High |
Precise differentiation of radical types |
Requires specialized equipment operation; samples need fresh preparation |
Fluorometric assays are the most common ROS detection method due to their flexibility and compatibility with instruments such as fluorescence microscopes, flow cytometers, and microplate readers. Researchers can select appropriate probes, such as DCFH-DA, DHE, or targeted variants, based on study goals, sample type, and available equipment.
03 Choosing a ROS Detection Protocol
3.1 For Overall Oxidative Stress Level Assessment: DCFH-DA Probe
It reacts with multiple ROS types (e.g., H2O2, ·OH) to produce green fluorescence, is compatible with various platforms (microscopy, flow cytometry), and provides a rapid readout of total ROS levels; perfect for initial drug screening or group comparisons.
3.2 For Studying Specific ROS Types: Specific Probes
For instance, mitochondrial-targeted probes emit red fluorescence specifically for superoxide, minimizing interference from other ROS/RNS. This is essential for research focused on mitochondrial dysfunction.
04 Matching Instruments to Your Goals
4.1 Microscopy (Fluorescence and Confocal)
Suitable for subcellular localization studies of ROS. Visualize ROS dynamics (e.g., during apoptosis) or organelle-specific distribution (e.g., mitochondrial superoxide) when paired with targeted probes.
4.2 Flow Cytometry
Enables quantitative, single-cell analysis of large populations. It supports statistical metrics (MFI, % positive cells) and multiparameter assays (e.g., ROS + apoptosis markers) to correlate ROS with phenotype.
4.3 Fluorescence Microplate Reader
Designed for high-throughput screening. Using 96-well plates, it efficiently compares multiple treatments or screens compound libraries.
05 Key Questions on DCFH-DA Detection
DCFH-DA, while user-friendly, requires attention to detail. Here are concise answers to common issues:
Q1: Optimal concentration and loading time?
A:Determine via pilot experiments. A common starting point is 10 µM for 30 minutes.
Q2:Does sample autofluorescence interfere? What is a typical signal range?
A:Green autofluorescence will interfere; other colors generally do not. Consider the red-fluorescent DHE probe if interference is suspected. Signal ranges vary by instrument; no universal reference exists.
Q3:Can it detect ROS in serum or tissue homogenates?
A:No. It is designed for live cells or fresh tissues processed into single-cell suspensions.
Q4:Tissue requirements for single-cell suspension?
A:Typically 0.1 g of tissue (aiming for 105-106 cells). Use enough digestion buffer to submerge the tissue.
Q5:Direct imaging of cells or tissues possible?
A:Yes for cells in dishes if using an inverted microscope. For tissues, process into a single-cell suspension first; for optimal detection, use a plate reader or flow cytometer instead of microscopy.
Q6:How to analyze microplate reader data?
A:This is a semi-quantitative assay. Directly compare relative fluorescence intensities between groups. Higher values indicate higher ROS levels.
Q7:Are controls needed for every experimental group?
A:If no background fluorescence is present, a single set of positive and negative controls using the normal or control group samples is sufficient.
Related Products
Table 2. ROS Detection Kits and Probes Overview
|
Target |
Product Name |
Probe |
Cat. No. |
Detection Instrument |
|
ROS |
Reactive Oxygen Species (ROS) Fluorometric Assay Kit (Green) |
DCFH-DA |
E-BC-K138-F |
Fluorescence microplate reader, Fluorescence microscope, Flow cytometry |
|
Reactive Oxygen Species (ROS) Fluorometric Assay Kit (Red) |
DHE |
E-BC-F005 |
||
|
Superoxide |
Mitochondrial Superoxide Fluorometric Assay Kit |
Specific Fluorescent Probe |
E-BC-F008 |
|
|
H2O2 |
Hydrogen Peroxide (H2O2) Fluorometric Assay Kit |
H2O2-specific Probe |
E-BC-F001 |
Fluorescence microplate reader |
Reference
[1] Manoharan RR, Prasad A, Pospíšil P, Kzhyshkowska J. ROS signaling in innate immunity via oxidative protein modifications. Front Immunol. 2024;15:1359600. Published 2024 Mar 7. doi:10.3389/fimmu.2024.1359600



