|
Purpose |
Sample |
Antibody Collocation |
|
|
Adjust the voltage |
1 |
Blank |
|
|
Adjust compensation |
2 |
CD3-PerCP/Cyanine5.5 |
|
|
3 |
CD4-Elab Fluor® 488 |
||
|
4 |
IL-17A-PE |
||
|
PE-FMO in combination with Isotype Control for auxiliary gating |
5 |
CD3-PerCP/Cyanine5.5, CD4-Elab Fluor® 488, Mouse IgG1, κ Isotype Control-PE |
|
|
Full panel |
6 |
CD3-PerCP/Cyanine5.5, CD4-Elab Fluor® 488, IL-17A- PE |
|
Marker |
Fluorochrome |
Clone No. |
Cat. No. |
|
CD3 |
PerCP/Cyanine5.5 |
OKT3 |
|
|
CD4 |
Elab Fluor® 488 |
SK3 |
|
|
IL-17A |
PE |
BL168 |
|
|
Mouse IgG1, κ Isotype Control |
PE |
MOPC-21 |
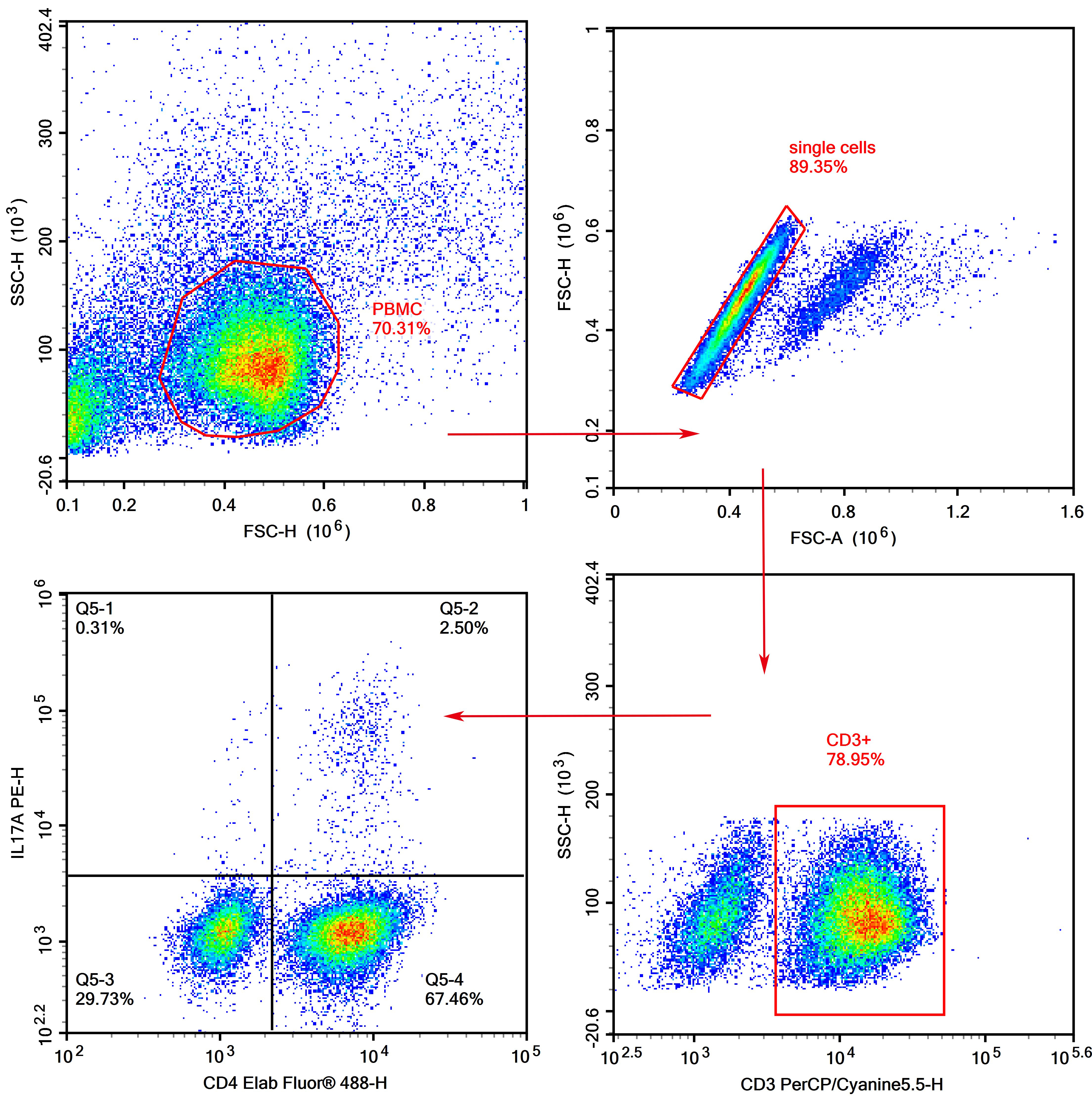
Tips:
1. After PBMC sorting, it is necessary to first use cytokine stimulating and blocking agents for stimulating and blocking culture (for stimulation-blocking experimental conditions, please refer to the instructions of the selected kit).
2. PMA stimulation can cause partial endocytosis of CD4 on the surface of human T cells, so we need to choose the CD4 clone SK3 with minimal impact on endocytosis.
3. Isotype control for IL-17A is necessary, since the expression of cytokines is generally not high.
4. CD3+CD4+ IL-17A+ is Th17 type.
5. The Permeabilization buffer may cause significant damage to cells, so it is recommended that the cell precipitates formed after centrifugation should be dispersed into cell suspensions before adding the Permeabilization buffer to reduce cell damage.



