Have you ever encountered these situations in your experiments: inexplicably high fluorescence background, unclear cell population separation, or expected positive and negative populations becoming indistinguishable? If these issues are troubling you, then it's time to focus on the most common interfering factor in flow cytometry experiments, dead cells.
Table of Contents
1. What effects can dead cells have on flow cytometry experiments?
2. How to effectively remove the influence of dead cells?
3. How to choose different samples?
01 What effects can dead cells have on flow cytometry experiments?
1. Non-specific binding: Dead cells exhibit increased membrane permeability and compromised integrity. Like "tiny sponges," they non-specifically bind to antibodies and fluorescent dyes, leading to non-specific staining and significantly elevated fluorescence background. This can obscure target positive population signals within a high background, resulting in increased false-positive rates and blurred cell population separation in experiment result.
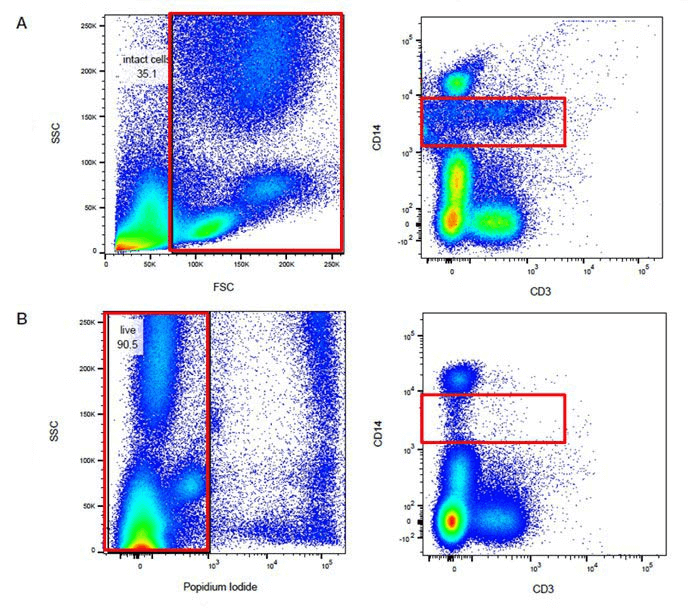
Fig. 1 Comparison of results of the same samples before and after excluding dead cells (Group A was not stained with dead or live dyes, while Group B was stained with PI).
2. Autofluorescence interference: Dead cells can cause enhanced background autofluorescence, with signal intensity potentially exceeding that of certain fluorescent dyes. This significantly impacts the detection of weakly expressed antigens or non-resolving markers. Moreover, the fluorescence emitted by dead cells lacks wavelength selectivity and can be detected across multiple channels, often appearing as diagonally distributed signals on dot plots, a key characteristic for identifying dead cell interference.
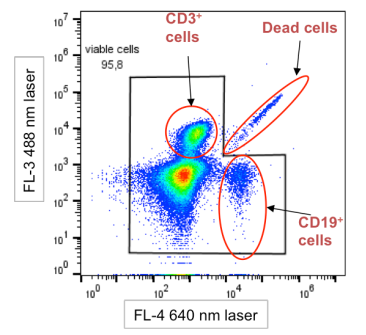
Fig. 2 Schematic diagram of dead cell interference characteristic. Dead cells exhibit a diagonal distribution pattern on the plot.
02 How to effectively remove the influence of dead cells?
The key to excluding dead cells lies in using viability dyes. Currently, the main viability dyes are categorized as nucleic acid stains and amine-reactive dyes, each based on distinct principles and suited for different application scenarios.
1. Nucleic acid dyes (Applicable to unfixed samples)
The principle of this method is based on the membrane integrity of live cells:
Live cells maintain intact membrane structure with selective permeability, preventing nuclear dyes such as PI, 7-AAD, and DAPI from entering the cell, thus exhibiting no fluorescence.
Dead cells have compromised membranes, allowing dyes to penetrate, bind to nucleic acids, and emit fluorescence.
For application, add the nucleic acid stain after flow cytometry antibody staining and incubate briefly before running samples on the flow cytometer.
Attention: If the experiment requires subsequent fixation, this process will compromise the membrane integrity of all cells, causing even live cells to display positive signals. Therefore, this method is not applicable when post-staining fixation is planned.
2. Amine-reactive dyes (Suitable for experiments requiring fixation)
The principle of this method is based on covalent binding between the dyes and protein primary amines:
Live cells exhibit weak fluorescence signals as the dyes only bind to surface proteins.
Dead cells show strong fluorescence signals due to compromised membranes, allowing dye penetration and binding to abundant intracellular proteins.
For application, perform viability staining before antibody staining. After washing, the cells can undergo fixation and permeabilization without loss of fluorescence signals.
03 How to choose different samples?
Not all samples require viability staining. Based on our experience, we provide the following practical recommendations:
1. Fresh peripheral blood or bone marrow samples: Generally contain few dead cells and may not require viability staining. However, if samples are not fresh or were improperly handled, staining is still recommended.
2. Spleen or lymph node tissues: These samples are relatively straightforward to prepare. The decision for viability staining can be based on single-cell suspension quality: proceed with staining if significant debris is observed under microscopy.
3. Difficult-to-prepare tissues or tumor samples: Typically contain abundant dead cells. Viability staining is strongly recommended.
4. Multicolor flow cytometry experiments: Viability staining is essential for these experiments. This is a critical step for reducing background and improving data quality. Ensure dye channel compatibility with your antibody panel.
Recommended Product
We offer a diverse range of nucleic acid stains and amine-reactive dyes to comprehensively support your needs: from basic surface marker detection to complex multicolor intracellular staining.
Table 1. Nuclear Staining and Cell Viability Detection Reagents
|
Product Name |
Cat. No. |
Size |
Application |
|
PI Reagent (50 μg/mL) |
E-CK-A161 |
50/100/200/500 T |
Nuclear Staining |
|
7-AAD Reagent (100 μg/mL) |
E-CK-A162 |
50/100/200/500 T |
|
|
DAPI Reagent (25 μg/mL) |
E-CK-A163 |
50/100/200/500 T |
|
|
STYX™ Green Fixable Viability Kit |
E-CK-A166 |
50/100/200 T |
Cell Viability Identification |
|
STYX™ Violet Fixable Viability Kit |
E-CK-A167 |
50/100/200 T |
Don't let dead cells obscure your real data. Proper use of viability dyes is a crucial step towards obtaining high-quality, reproducible flow cytometry results. Follow Elabscience® for more expert knowledge on flow cytometry experiments.



