The occurrence of ferroptosis primarily depends on the accumulation of iron ions and lipid peroxidation in the body. Cells undergoing ferroptosis display distinct morphological differences compared to other forms of cell death such as apoptosis and autophagy, with particularly notable changes in mitochondrial morphology. As a crucial organelle, mitochondria play a key role in various cell death pathways, including apoptosis, necroptosis, and pyroptosis, with clear evidence of their deep involvement. Whether mitochondria directly participate in ferroptosis is still under investigation, but a growing body of research indicates that mitochondrial ROS, iron, energy metabolism, and other pathways are closely related to the ferroptosis process.
Table of Contents
1. Mitochondrial ROS and Ferroptosis
2. Mitochondrial Iron Metabolism and Ferroptosis
3. Mitochondrial Energy Metabolism and Ferroptosis
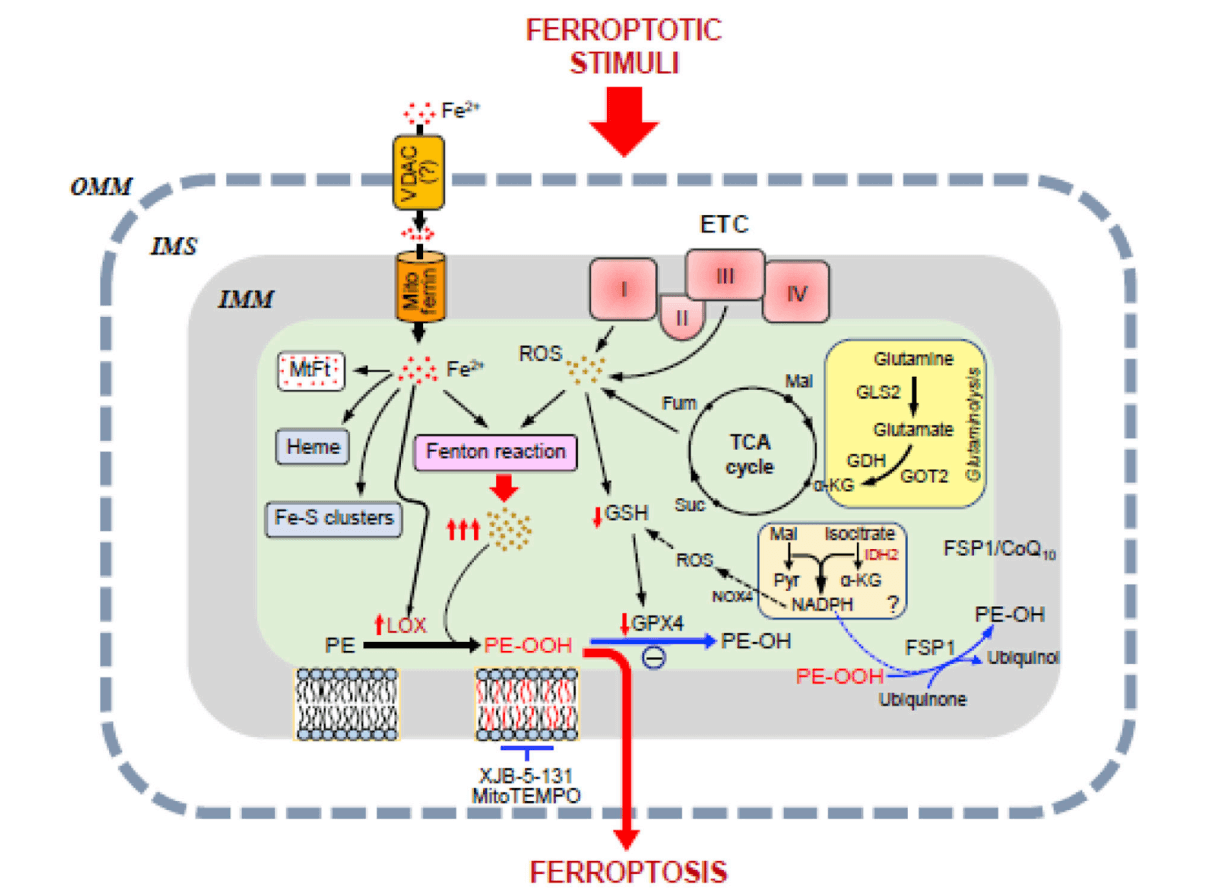
Fig. 1 Potential pathways of mitochondrial involvement in ferroptosis[1].
01 Mitochondrial ROS and Ferroptosis
Reactive oxygen species (ROS) show a strong correlation with ferroptosis, and mitochondria are one of the main sources of ROS. The primary cause of cell death in ferroptosis is the massive production of lipid peroxides, ultimately leading to membrane damage. The ferroptosis inducer RSL3 inhibits glutathione peroxidase 4 (GPX4). After RSL3-induced ferroptosis in HT22 cells and mouse embryonic fibroblasts, adding the mitochondria-targeted antioxidant Mitoquinone (MitoQ) enhances mitochondrial integrity and reduces ferroptosis[2]. The ferroptosis activator Erastin inhibits voltage-dependent anion channels (VDAC2/VDAC3), accelerates oxidation, and leads to the accumulation of endogenous ROS. When Erastin or RSL3 induces ferroptosis in SK-Hep1ρ+ cells, mitochondrial ROS increases significantly; this ferroptosis is markedly suppressed by the addition of MitoQ[3]. Multiple studies have shown that using mitochondria-targeted ROS scavengers can inhibit ferroptosis in various cell types and improve mitochondrial membrane integrity. These findings suggest that increased mitochondrial ROS promotes ferroptosis, while clearing mitochondrial ROS can inhibit it.
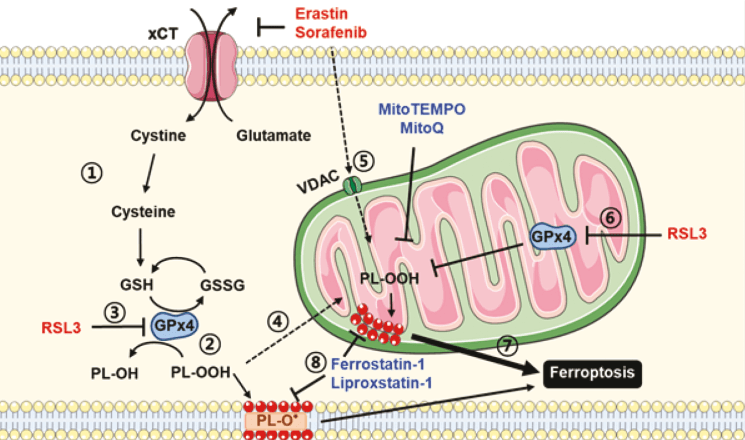
Fig. 2 Ferroptosis and mitochondrial ROS accumulation[3].
02 Mitochondrial Iron Metabolism and Ferroptosis
Iron metabolism within mitochondria itself can also regulate ferroptosis. Mitochondria contain about 20-50% of the cell's total iron. Iron-containing proteins in mitochondria serve as essential cofactors in enzymatic redox reactions for electron transfer. Mitochondrial free iron overload can increase mtROS levels via the Fenton reaction, activate mitochondrial NOX4 and 15-lipoxygenase, leading to phospholipid peroxidation and ferroptosis. Overexpression of mitochondrial ferritin (FtMt) in SH-SY5Y cells improves mitochondrial iron homeostasis and inhibits Erastin-induced ferroptosis[4]. CISD1 is an iron‑sulfur cluster protein located on the mitochondrial outer membrane. Downregulation of CISD1 increases iron‑mediated lipid peroxidation in mitochondria. Conversely, CISD1 overexpression reduces mitochondrial iron uptake and lipid peroxidation, and suppresses Erastin‑induced ferroptosis. These findings indicate that mitochondrial iron metabolism plays a significant role in lipid oxidation and the ferroptosis process[5].
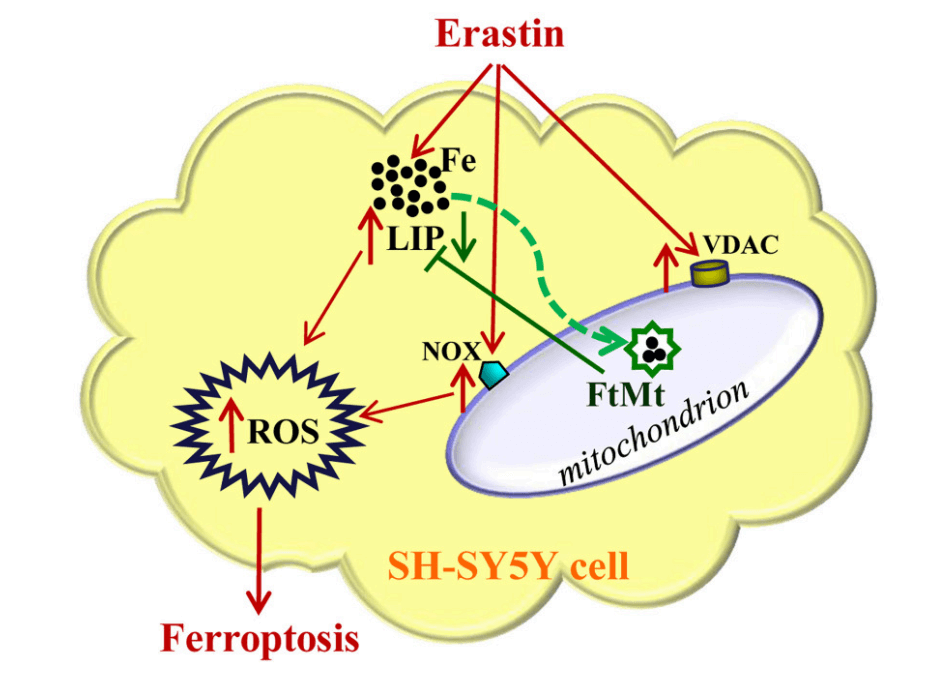
Fig. 3 Mitochondrial iron metabolism and ferroptosis[4].
03 Mitochondrial Energy Metabolism and Ferroptosis
Mitochondrial energy metabolism is also linked to ferroptosis. As the central hub for cellular energy conversion, mitochondria house multiple enzymes involved in respiration that may participate in ferroptosis regulation, such as aconitase, citrate synthase, α‑ketoglutarate dehydrogenase, fumarate hydratase, and NADPH oxidase 4. These mitochondrial regulatory enzymes mainly influence ferroptosis by affecting mitochondrial ROS production, leading to lipid peroxidation[6]. Coenzyme Q10 is a crucial electron carrier in the mitochondrial electron transport chain and a lipophilic antioxidant. Recent studies have found that the ferroptosis suppressor protein FSP1 can reduce CoQ10, thereby inhibiting ferroptosis. FSP1 operates independently of the GPX4 antioxidant system, participating in an alternative pathway that counteracts lipid peroxidation in ferroptosis[7]. Mitochondria are involved in numerous metabolic pathways; how these pathways affect ferroptosis requires further exploration.
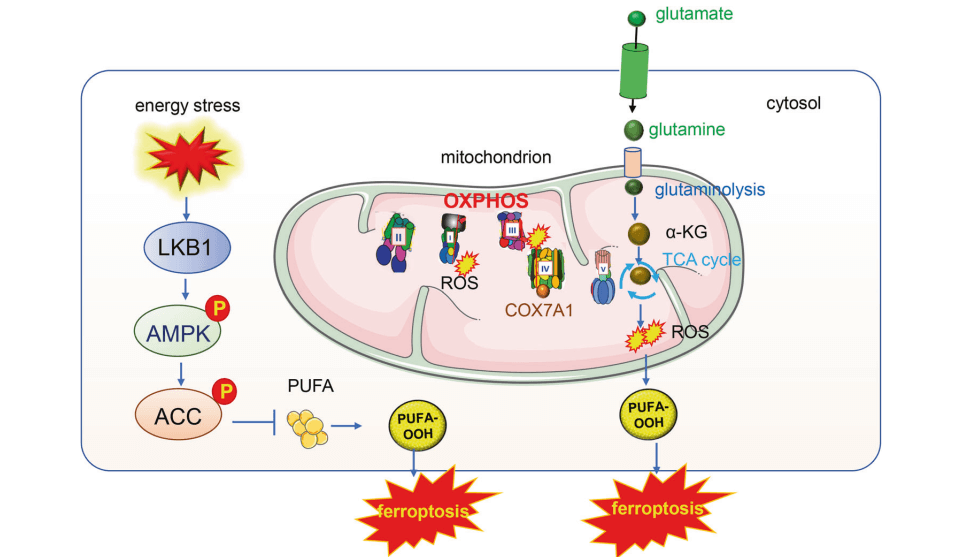
Fig. 4 Mitochondrial energy metabolism and ferroptosis[8].
The specific role of mitochondria in ferroptosis and the pathways through which mitochondria mediate ferroptosis signaling remain debated. However, increasing evidence in recent years indicates a close relationship between mitochondria and ferroptosis. Changes in the levels of substances such as ROS and iron within mitochondria can influence the ferroptosis process. In summary, the precise regulatory mechanisms of mitochondria in ferroptosis warrant continued research and exploration.
References:
1. Javadov S. Mitochondria and ferroptosis[J]. Current Opinion in Physiology, 2022, 25: 100483.
2. Jelinek, Anja, Heyder, et al. Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis[J]. Free Radical Biology & Medicine the Official Journal of the Oxygen Society, 2018.
3. Oh, SJ., Ikeda, M., Ide, T., et al. Mitochondrial event as an ultimate step in ferroptosis. Cell Death Discovery. 8, 414 (2022).
4. Wang Y Q, Chang S Y, Wu Q, et al. The protective role of mitochondrial ferritin on erastin-induced ferroptosis[J]. Frontiers in Aging Neuroscience, 2016, 8: 308.
5. Javadov, S.. Mitochondria and ferroptosis. Current Opinion in Physiology, 25. (2022)
6. Gao M, Yi J, Zhu J, et al. Role of mitochondria in ferroptosis. Mol Cell, 2019, 73(2): 354-363 e353
7. Bersuker K, Hendricks J, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature, 2019, 575(7784): 688-692
8. Liu Y, Lu S, Wu L, et al. The diversified role of mitochondria in ferroptosis in cancer[J]. Cell Death & Disease, 2023, 14(8): 519.



