Interleukin 6 (IL-6) is widely recognized as a multifunctional cytokine, attributed to its pleiotropic roles in both physiological homeostasis and pathological progression. Under physiological conditions, IL-6 maintains extremely low basal concentrations in peripheral blood and interstitial fluids. By contrast, during aging, chronic inflammation, or other pathological states (with the liver representing a key affected organ) IL-6 levels undergo marked elevation, where it exerts pivotal roles in driving inflammatory responses, tissue fibrosis, and carcinogenesis[1]. As a core inflammatory biomarker, IL-6 is indispensable across diverse scientific research paradigms, including the elucidation of inflammatory mechanisms, the screening of diagnostic biomarkers for disease stratification, and the validation of the anti-inflammatory efficacy of novel therapeutic agents. Notably, the accurate quantification of IL-6 is directly correlated with the robustness and reproducibility of experimental conclusions. In this context, the High-Sensitivity ELISA Assay for IL-6 Detection emerges as an indispensable research tool to fulfill this critical analytical requirement.
This article reviews the transduction mechanism of the IL-6 signaling pathway, dysregulation of the IL-6 signaling pathway and its association with disease pathogenesis, applications of the high-sensitivity IL-6 ELISA kit in disease-related research, and comparative analyses of high sensitivity ELISA versus traditional ELISA methodologies.
Table of Contents
1. Introduction to IL-6 Structure and Function
2. Transduction Mechanisms of the IL-6 Signaling Pathway
3. Dysregulation of IL-6 Signaling in Disease Pathogenesis
4. Applications of High-Sensitivity IL-6 ELISA Kit in Diseases
5. High-Sensitivity ELISA vs Traditional ELISA
01 Introduction to IL-6 Structure and Function
IL-6 is a multifunctional glycoprotein consisting of 188 amino acid residues, modified by N-linked glycosylation, with a molecular weight ranging from 21 to 28 kDa. It possesses a conserved four-helix bundle structure, a hallmark signature of the IL-6 cytokine family[2]. Predominantly synthesized by immune cells and a broad spectrum of parenchymal cells, IL-6 orchestrates systemic biological responses via the activation of specific receptor-mediated signaling cascades. As a pivotal signaling molecule, it exerts indispensable roles in mediating human immune defense, orchestrating acute-phase reactions, and facilitating tissue repair processes. Under physiological conditions, IL-6 is essential for eradicating pathogenic infections, promoting antibody synthesis, and accelerating post-traumatic tissue recovery. Conversely, its persistent overproduction confers pathogenic properties, thereby acting as a critical driver of chronic inflammatory disorders, autoimmune diseases (e.g., rheumatoid arthritis), cytokine storm syndromes during severe infections, and the progression of diverse malignancies.
02 Transduction Mechanisms of the IL-6 Signaling Pathway
IL-6 is expressed on the cell membrane of specific cell populations, including hepatocytes, immune cells, and certain endothelial cells[3]. The biological functions of IL-6 are tightly dependent on its receptor complex, which comprises the specific ligand-binding subunit IL-6R (IL-6 receptor) and the universal signal-transducing component gp130. The signaling cascade of IL-6 is intricate and is primarily categorized into three distinct modalities: classical signaling, trans-signaling, and cluster signaling. Collectively, these signaling modes orchestrate the diverse physiological activities of IL-6[4]. The detailed mechanisms underlying these three signaling modalities are elaborated as follows:
2.1 Classical Signaling
In this signaling mode, IL-6 engages with the membrane-bound interleukin-6 receptor (IL-6R) through its conserved binding domain (site I), thereby permitting the sequential interaction of IL-6 domains (sites II and III) with the glycoprotein 130 (gp130) coreceptor. This receptor-ligand interaction induces gp130 dimerization and subsequent activation of downstream intracellular signaling cascades. Notably, the specific binding of IL-6 site I to IL-6R constitutes an indispensable prerequisite for the initiation of this entire signaling pathway.
2.2 Trans-signaling
When IL-6R undergoes proteolytic cleavage by enzymes such as ADAM17, it generates a soluble isoform (sIL-6R) that retains the capacity to bind to the conserved site I of IL-6. The resultant IL-6/sIL-6R complex subsequently engages with gp130 expressed on the surface of adjacent cells via IL-6 sites II and III, thereby triggering downstream signaling cascades even in cell types that exclusively express gp130 (but lack membrane-bound IL-6R)
2.3 Cluster Signaling
This mechanism is mediated by intercellular crosstalk: IL-6 first binds to IL-6R expressed on the surface of cell A (e.g., dendritic cells) via its conserved site I. Subsequently, the formed IL-6/IL-6R complex interacts with gp130 anchored on the membrane of cell B (e.g., T cells) through IL-6 sites II and III, thereby allowing cell A to function as a signaling initiator that elicits downstream signaling cascades in cell B
A key shared characteristic of both IL-6 trans-signaling and cluster signaling is their capacity to render cells expressing only gp130 (but lacking IL-6R) responsive to IL-6 stimulation. This unique attribute substantially broadens the spectrum of potential IL-6 target cells and further amplifies the functional versatility of IL-6 in orchestrating immune regulation and mediating inflammatory responses.
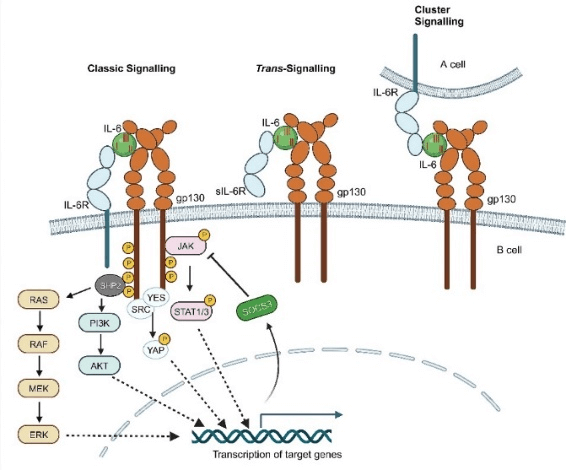
Fig. 1 Schematic diagram of IL-6 signaling pathways: Classical, Trans-, and Cluster signaling[4].
03 Dysregulation of IL-6 Signaling in Disease Pathogenesis
Under physiological conditions, a delicate equilibrium is maintained between IL-6 classical signaling and trans-signaling, which cooperatively sustains cellular and systemic homeostasis[5]. In contrast, under pathological states, the trans-signaling pathway undergoes excessive activation and emerges as the dominant signaling modality. Given its capacity to activate nearly all gp130-expressing cells, the functional scope of trans-signaling far surpasses that of classical signaling. This shift converts IL-6 signaling from a tightly regulated, physiologically protective cascade into an unconstrained, pathologically detrimental process,a pivotal mechanism underlying the pathogenesis of autoimmune diseases, chronic inflammatory disorders, malignancies, metabolic syndromes, and cytokine storm syndromes[6].
3.1 IL-6 and Autoimmune Diseases
IL-6 exerts a central, pivotal pathogenic role in autoimmune disorders, predominantly via its trans-signaling pathway. The core mechanism underlying its disease-promoting effects involves the induction of a self-perpetuating inflammatory cycle: IL-6 impairs immune tolerance by dual means,on the one hand, it promotes autoantibody secretion in B lymphocytes, and on the other hand, it disrupts T-cell homeostasis by enhancing the differentiation of pro-inflammatory T helper 17 (Th17) cells while concomitantly suppressing the immunosuppressive function of regulatory T (Treg) cells. Simultaneously, IL-6 broadly amplifies innate immune-mediated inflammation by triggering systemic acute-phase responses, recruiting inflammatory cell infiltration into target tissues, and directly contributing to tissue damage (e.g., bone erosion in rheumatoid arthritis). This tissue injury, in turn, further stimulates local IL-6 release, thereby establishing a critical pathogenic feedback loop that sustains chronic inflammation and abrogates its resolution[7].

Fig. 2 Mechanism of Action of IL-6 in Immunity[2].
Table 1. Spectrum of Autoimmune Diseases
|
Level of Association |
Diseases |
Core Link and Evidence |
|
Core/Classic Diseases (Targeted Therapies Approved) |
• Rheumatoid Arthritis • Giant Cell Arteritis / Polymyalgia Rheumatica • Systemic Juvenile Idiopathic Arthritis • Castleman Disease (Idiopathic Multicentric Type) |
IL-6 is a widely recognized core pathogenic factor. Targeted therapies against IL-6/IL-6R (e.g., tocilizumab) are standard treatment options, directly validating its central role. |
|
Diseases with Clearly Established Involvement (Well-Defined Pathogenic Mechanisms) |
• Systemic Lupus Erythematosus • Takayasu Arteritis • Adult-Onset Still’s Disease |
IL-6 serves as a key pathogenic mediator and correlates closely with disease activity. Targeted therapies have exhibited marked efficacy and represent a major treatment option. |
|
Related/Associated Diseases(Involvement in Pathogenic Processes)Involvement (Well-Defined Pathogenic |
• Sjögren’s Syndrome • Systemic Sclerosis • IgG4-Related Disease • Certain Autoimmune Cytopenias |
IL-6 participates in distinct pathogenic processes (e.g., inflammation, fibrosis, immune dysregulation) and constitutes a key component of the complex disease network. |
3.2 The Association of IL-6 with Tumor Diseases
The IL-6/JAK/STAT3 signaling pathway functions as a central regulatory axis in the progression, immune evasion, and chemoresistance of multiple malignancies[8]. Persistently hyperactivated in tumors, this pathway drives cancer cell proliferation, survival, angiogenesis, and metastasis via remodeling of the tumor microenvironment (TME). Upon ligation to its receptor complex, IL-6 activates JAK kinases, which phosphorylate STAT3 to form homodimers that translocate into the nucleus. This event promotes STAT3-dependent transcription of oncogenes and fosters an immunosuppressive TME, characterized by infiltration of tumor-associated macrophages (TAMs), cancer-associated fibroblasts (CAFs), and regulatory T cells (Tregs), collectively mediating immune escape. Moreover, this signaling axis enhances tumor cell adaptation to environmental stresses (e.g., hypoxia, nutrient deprivation) through regulation of metabolic reprogramming pathways including glycolysis and glutathione metabolism.
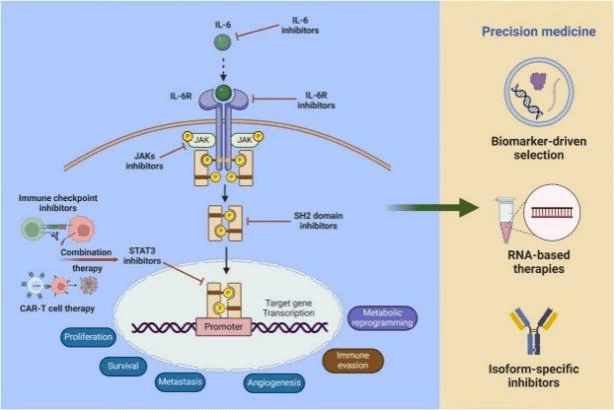
Fig. 3 Mechanism of action of the IL-6/JAK/STAT3 signaling pathway in tumors[8].
3.3 IL-6 and Cardiovascular Diseases
Among inflammatory mediators, IL-6 represents a key contributor to the pathophysiology of cardiovascular diseases. Synthesized by macrophages, monocytes, endothelial cells, vascular smooth muscle cells, and fibroblasts, this cytokine plays a critical role in driving multiple pathological processes of atherosclerotic cardiovascular disease (ASCVD). In response to cholesterol accumulation and oxidative stress, pro-inflammatory cytokines further recruit immune cells (e.g., macrophages, T cells, B cells) and stimulate the secretion of additional pro-inflammatory mediators (including IL-6), thereby activating endothelial cells and upregulating the expression of cell adhesion molecules. IL-6 also promotes macrophage uptake of oxidized low-density lipoprotein, facilitating foam cell formation. Furthermore, it stimulates the proliferation and migration of smooth muscle cells and enhances matrix secretion, accelerating atherosclerotic plaque progression. IL-6 destabilizes plaques by exacerbating local inflammation, thinning the fibrous cap, and impairing collagen synthesis, rendering plaques more susceptible to rupture. This plaque instability is further exacerbated by IL-6-induced tissue factor expression, which fosters a pro-thrombotic microenvironment.
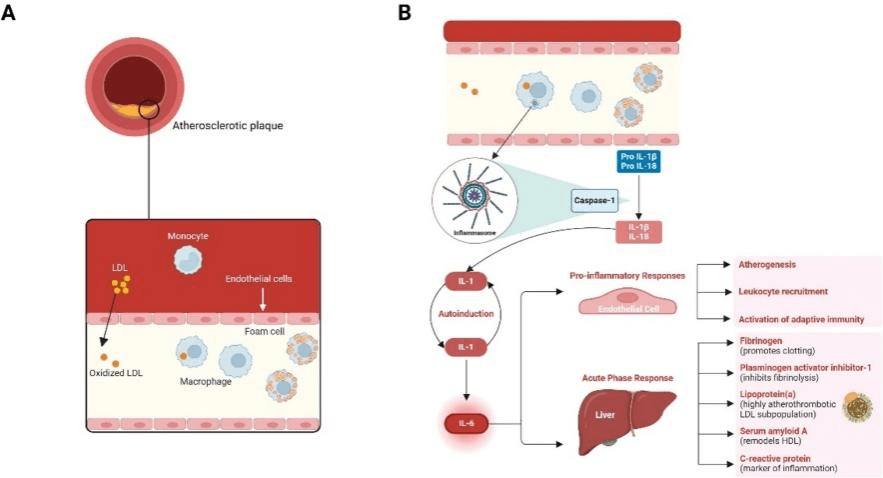
Fig. 4 Pathogenesis of Atherosclerotic Cardiovascular Disease (ASCVD)[9].
04 Applications of High Sensitivity IL-6 ELISA Kit in Diseases
Having delineated the pivotal roles of IL-6, clinicians and researchers are confronted with a core question: how to accurately and stably quantify this critical biomarker? This is because subtle fluctuations in low IL-6 concentrations often hold the key to early inflammatory alerts, precise disease activity assessment, and treatment response monitoring.
High Sensitivity ELISA: As a highly precise pharmacodynamic indicator, this technique enables accurate quantification of even minute changes in IL-6 levels (e.g., a reduction from 0.8 pg/mL to 0.3 pg/mL), thereby markedly improving the efficiency and success rate of drug development. A direct comparison with the lower detection limit of traditional ELISA reveals that high-sensitivity ELISA is not merely marginally more sensitive,it represents a qualitative technological leap. It extends IL-6 detection from the "pathological level" to the realms of "physiological regulation and early pathological changes", empowering researchers to explore fine-scale biological regulatory mechanisms and the early pathogenic origins of diseases within concentration ranges previously beyond analytical reach.
4.1 Application of High Sensitivity IL-6 in Autoimmune Diseases
In autoimmune diseases, high-sensitivity IL-6 ELISA kits act as a monitoring tool for "deep remission" and a precise metric for targeted therapy management. When evaluating the efficacy of IL-6-targeted agents (e.g., tocilizumab) and verifying the achievement of complete target inhibition, quantification via ELISA kits is indispensable. For assessing subclinical or low-grade inflammation in disorders like rheumatoid arthritis and systemic lupus erythematosus, high-sensitivity il-6 elisa assay offer superior sensitivity over traditional ELISA kits.
4.2 Application of High Sensitivity IL-6 in Cardiovascular Diseases
In cardiovascular diseases, the high-sensitivity IL-6 ELISA kit acts as a "radar" for occult vascular inflammation and an early-warning indicator for long-term risks, with primary utility in risk assessment and prognostic stratification:
In asymptomatic individuals or stable-phase patients, mild IL-6 elevation acts as an independent predictor of future cardiovascular events (e.g., myocardial infarction, stroke).
It serves to assess the inflammatory burden of atherosclerotic lesions.
4.3 Application of High Sensitivity IL-6 in Tumor Diseases
Oncology Applications: The high-sensitivity IL-6 ELISA kit generates ELISA results (or ELISA test results) that serve as an indicator of tumor microenvironment immune status and a supplementary tool for prognostic evaluation.
Prognostic Assessment: IL-6 levels correlate with tumor malignancy, clinical stage, and poor prognosis across multiple cancer types (e.g., hepatocellular carcinoma, breast cancer, ovarian cancer, lymphoma)
ELISA positive IL-6 elevation is indicative of enhanced immunosuppression within the tumor microenvironment, a factor that may compromise immunotherapy efficacy.
Therapeutic Efficacy Monitoring and Drug Resistance Alerting:
Quantify dynamic IL-6 level changes during targeted therapy or chemotherapy to evaluate treatment responses.
Sustained IL-6 elevation may signal the development of drug resistance or disease progression.
05 High Sensitivity ELISA vs Traditional ELISA
The core advantage of high sensitivity ELISA over traditional ELISA lies in its 10-1000-fold higher detection sensitivity. By virtue of signal amplification and systematic optimization, high-sensitivity ELISA attains sensitivity levels comparable to chemiluminescence assays while retaining operational simplicity, rendering it an ideal tool for detecting low-abundance proteins. Traditional ELISA can be analogized to a "telescope," which excels at visualizing distinct, well-defined targets; in contrast, high-sensitivity ELISA functions as a "microscope," uncovering subtle traces obscured within baseline signals. These two techniques are mutually complementary, collectively underpinning diverse stages of disease diagnosis and clinical management.
Table 2. High Sensitivity ELISA vs Traditional ELISA
| Comparison Dimension |
Traditional ELISA Kit |
High Sensitivity ELISA Kit |
Core Advantages of High Sensitivity Kit |
|
Detection Sensitivity |
Relatively low detection at the level of pg/mL (e.g1-10 pg/mL) |
Extremely high, capable of reaching fg/mL level (e.g., 0.01-0.1 pg/mL), 10-100 times more sensitive than conventional kits |
Enables detection of very low-concentration target molecules, suitable for trace samples or low-expression scenarios |
|
Detection Range |
Broad range, suitable for medium to high concentration samples |
Usually narrower range, focusing on accurate quantification in low-concentration intervals |
Provides higher resolution and accuracy in the low-concentration range |
|
Application Scenarios |
1) Detection of markedly elevated biomarkers during active disease phases 2) Detection of high-concentration factors in cell culture supernatants and serum(e.g il 6 levels in serum) 3) Preliminary screening or Preliminary screening or qualitative/semi-quantitative analysis |
1) Monitoring of low-grade/residual inflammation (e.g., during remission of autoimmune diseases) 2) Detection of early or mild pathological changes (e.g., subclinical cardiovascular risk) 3) Detection in trace samples such as cerebrospinal fluid, saliva, and tissues 4) Pharmacodynamic studies (evaluation of complete target inhibition by targeted drugs) |
Achieves "earlier, subtler, and more precise" detection, supporting in-depth disease management and cutting-edge research |
|
Technical Features |
1) Traditional enzyme-based amplification system 2) Relatively simple operation with standardized procedures |
1) Incorporates signal amplification technologies (e.g.,electrochemiluminescence, antibody cascade amplification, high-affinity antibody pairs) 2) Optimized antibody affinity with stricter background noise control |
Breaks through the lower detection limit through technological innovation, providing higher signal-to-noise ratio and repeatability |
|
Sample Requirements |
Lower requirements for sample dilution, relatively adaptable |
Often requires avoiding excessive dilution and is more sensitive to sample matrix effects (e.g., interference from hemolysis or lipemia) |
More suitable for direct detection of trace targets in original samples, reducing errors introduced by dilution |
Recommended Elabscience® High Sensitivity ELISA Kits
Leveraging the unique advantages of High Sensitivity ELISA technology, Elabscience® has developed a series of high-performance High Sensitivity ELISA designed to provide researchers worldwide with sensitive, stable, and ready-to-use solutions, aiding in more efficient unraveling of immune responses.
Core Advantages of Elabscience® Products:
Compatible with Multiple Samples: Supports human/mouse PBMCs, splenocytes, TILs, and other cell samples.
Multi-Species and Multiplex Coverage: Includes human and mouse species, targeting various key cytokines/factors such as IFN gamma, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-17A, TNF-α, and others.
Flexible Experiment Compatibility: Pre-coated and ready-to-use, with detachable strips for diverse needs.
Superior Detection Performance: Intra- and inter-batch CV < 10%, with >99% data accuracy verified.
Worry-Free Service: One-on-one service with a 24-hour response.
Elabscience® High Sensitivity of Popular Products
Table 3. Research Tools for cytokines
|
Cat. No. |
Product Name |
Size |
|
E-HSEL-H0003 |
High Sensitivity Human IL-6 (Interleukin 6) ELISA Kit |
96 T*1/96 T*5 |
|
E-HSEL-M0003 |
High Sensitivity Mouse IL-6 (Interleukin 6) ELISA Kit |
96 T*1/96 T*5 |
|
E-HSEL-H0005 |
High Sensitivity Human IL-10 (Interleukin 10) ELISA Kit |
96 T*1/96 T*5 |
|
E-HSEL-M0009 |
High Sensitivity Mouse TNF-α (Tumor Necrosis Factor Alpha) ELISA Kit |
96 T*1/96 T*5 |
|
E-HSEL-R0002 |
High Sensitivity Rat IL-1β (Interleukin 1 Beta) ELISA Kit |
96 T*1/96 T*5 |
|
E-HSEL-M0001 |
High Sensitivity Mouse IL-1β (Interleukin 1 Beta) ELISA Kit |
96 T*1/96 T*5 |
|
E-HSEL-H0002 |
High Sensitivity Human IL-2 (Interleukin 2) ELISA Kit |
96 T*1/96 T*5 |
|
E-HSEL-M0002 |
High Sensitivity Mouse IL-4 (Interleukin 4) ELISA Kit |
96 T*1/96 T*5 |
|
E-HSEL-M0007 |
High Sensitivity Mouse IFN-γ (Interferon Gamma) ELISA Kit |
96 T*1/96 T*5 |
Reference
[1] Al-Khayri, J.M., et al., Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules, 2022. 27(9).
[2] Hirano, T., IL-6 in inflammation, autoimmunity and cancer. Int Immunol, 2021. 33(3): p. 127-148.
[3] Ene, C.V., et al., IL-6 Signaling Link between Inflammatory Tumor Microenvironment and Prostatic Tumorigenesis. Anal Cell Pathol (Amst), 2022. 2022: p. 5980387.
[4] Wang, M.J., et al., The double-edged effects of IL-6 in liver regeneration, aging, inflammation, and diseases. Exp Hematol Oncol, 2024. 13(1): p. 62.
[5] Rose-John, S., Blocking only the bad side of IL-6 in inflammation and cancer. Cytokine, 2021. 148: p. 155690.
[6] Rose-John, S., Local and systemic effects of interleukin-6 (IL-6) in inflammation and cancer. FEBS Lett, 2022. 596(5): p. 557-566.
[7] Tanaka, T., M. Narazaki, and T. Kishimoto, Interleukin (IL-6) Immunotherapy. Cold Spring Harb Perspect Biol, 2018. 10(8).
[8] Thuya, W.L., et al., Insights into IL-6/JAK/STAT3 signaling in the tumor microenvironment: Implications for cancer therapy. Cytokine Growth Factor Rev, 2025. 85: p. 26-42.
[9] Mehta, N.N., E. deGoma, and M.D. Shapiro, IL-6 and Cardiovascular Risk: A Narrative Review. Curr Atheroscler Rep, 2024. 27(1): p. 12.



