Mitochondria exerts dual pivotal roles in the intricate interplay between cancer and metabolism, sustaining basal energy production while driving the metabolic reprogramming essential for tumor progression. Contrary to the classical Warburg hypothesis, functional mitochondria remain indispensable to the majority of tumors, supporting biosynthetic processes, redox signaling, and metabolic adaptability within the nutrient-depleted tumor microenvironment.
In this issue, we systematically summarize mitochondrial-associated metabolic reprogramming mechanisms, key biomarkers of mitochondrial dysfunction, core functional assessment assays, advanced analytical techniques, and emerging therapeutic strategies targeting mitochondrial vulnerabilities.
Table of Contents
1. Role of mitochondria in cancer metabolism
2. Metabolic reprogramming in cancer: the mitochondrial connection
3. Biomarkers of mitochondrial dysfunction in cancer
4. Assays for mitochondrial function in cancer research
5. Advanced techniques for analyzing mitochondrial metabolism in cancer cells
6. Therapeutic strategies targeting mitochondrial dysfunction in cancer
01 Role of mitochondria in cancer metabolism
Mitochondria function as central integrator of cancer cell energy metabolism. It present a remarkable metabolic flexibility, allowing cancer cells to dynamically balance glycolysis (the Warburg effect) and oxidative phosphorylation (OXPHOS) to meet fluctuating energetic and biosynthetic demands, a plasticity essential for thriving in the challenging tumor microenvironment (TME) characterized by nutrient deprivation and hypoxia[1].Acting as pivotal biosynthetic hubs, mitochondria host a reprogrammed tricarboxylic acid (TCA) cycle that generates key intermediates (e.g., citrate for lipid synthesis) and contributes to NADPH production, thereby fuelling the anabolic processes required for rapid proliferation[2]. As the primary source of reactive oxygen species (ROS), mitochondria critically regulate redox signaling. Moderate ROS levels can drive pro-tumorigenic pathways, while excessive ROS can induce cell death, underscoring their Janus-faced role in cancer biology[3] . Furthermore, mitochondria are engines of metabolic adaptability, enabling cancer cells to utilize diverse fuel sources such as glutamine and fatty acids through pathways like reductive carboxylation and anaplerosis, which are vital for survival under nutrient stress[4]. This intricate involvement makes targeting mitochondrial processes a promising strategic frontier in cancer cell energy metabolism for therapeutic intervention.
Notably, secondary mitochondrial dysfunction, often involving alterations in mitochondrial DNA (mtDNA) integrity, dynamics (fusion and fission), and respiratory chain activity, is a hallmark of many cancers[5].This dysfunction creates unique metabolic dependencies (e.g., arginine auxotrophy, addiction to fatty acid synthesis) that represent promising therapeutic vulnerabilities.
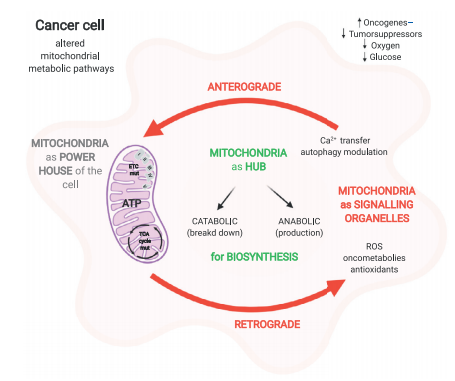
Fig. 1 Overview of the central role of mitochondria in cell metabolism[6].
02 Metabolic reprogramming in cancer: The mitochondrial connection
Building on the central role of mitochondria in cancer metabolism , metabolic reprogramming, an established hallmark of cancer, relies heavily on mitochondrial adaptability to support malignant progression. While the Warburg effect (enhanced aerobic glycolysis) is historically interpreted as evidence of mitochondrial dysfunction[1], accumulating evidence confirms that functional mitochondria remain indispensable for supporting biosynthetic pathways and redox homeostasis in cancer cells.
Key aspects of mitochondrial-linked metabolic reprogramming include:
Glucose metabolism reprogramming: Glucose metabolism reprogramming is a central feature of cancer and glucose metabolism, characterized by the upregulation of key glycolytic enzymes such as hexokinase 2 (HK2) and pyruvate kinase M2 (PKM2), while maintaining mitochondrial TCA cycle activity to generate essential biosynthetic precursors[1]. This 'glycolytic-TCA shuttle' ensures dual metabolic advantages: rapid ATP production via glycolysis and sustained anabolic metabolism through mitochondrial intermediates.
Lipid metabolism dysregulation: The dysregulation of cellular fatty acid metabolism and cancer is a established hallmark, where cancer cells co-opt both mitochondrial β-oxidation and fatty acid synthase (FASN)-mediated de novo fatty acid synthesis to meet the heightened demands for membrane biogenesis, energy production, and lipid-based signaling molecules essential for proliferation and survival .This metabolic reprogramming is particularly critical in aggressive malignancies, including specific subtypes such as metabolic lung cancer and triple-negative breast cancer[7].
Amino acid metabolism reprogramming: The dysregulation of arginine metabolism cancer is increasingly recognized as a hallmark of tumor progression and immune evasion. Mitochondrial arginase 1 (ARG1)-mediated arginine catabolism within the tumor microenvironment drives immunosuppression and supports tumor growth. Conversely, therapeutic arginine deprivation exploits the metabolic vulnerability of ASS1-deficient cancers, inducing severe mitochondrial dysfunction and ultimately triggering cancer cell death[8].
TCA Cycle reprogramming: Mutations in TCA enzymes drive tumorigenesis: succinate dehydrogenase (SDH) loss leads to succinate accumulation, stabilizing HIF-1α; fumarate hydratase (FH) deficiency results in fumarate-driven EMT; and mutant isocitrate dehydrogenase (IDH) produces D-2-hydroxyglutarate(D-2HG), which disrupts epigenetic regulation and drives malignant transformation.
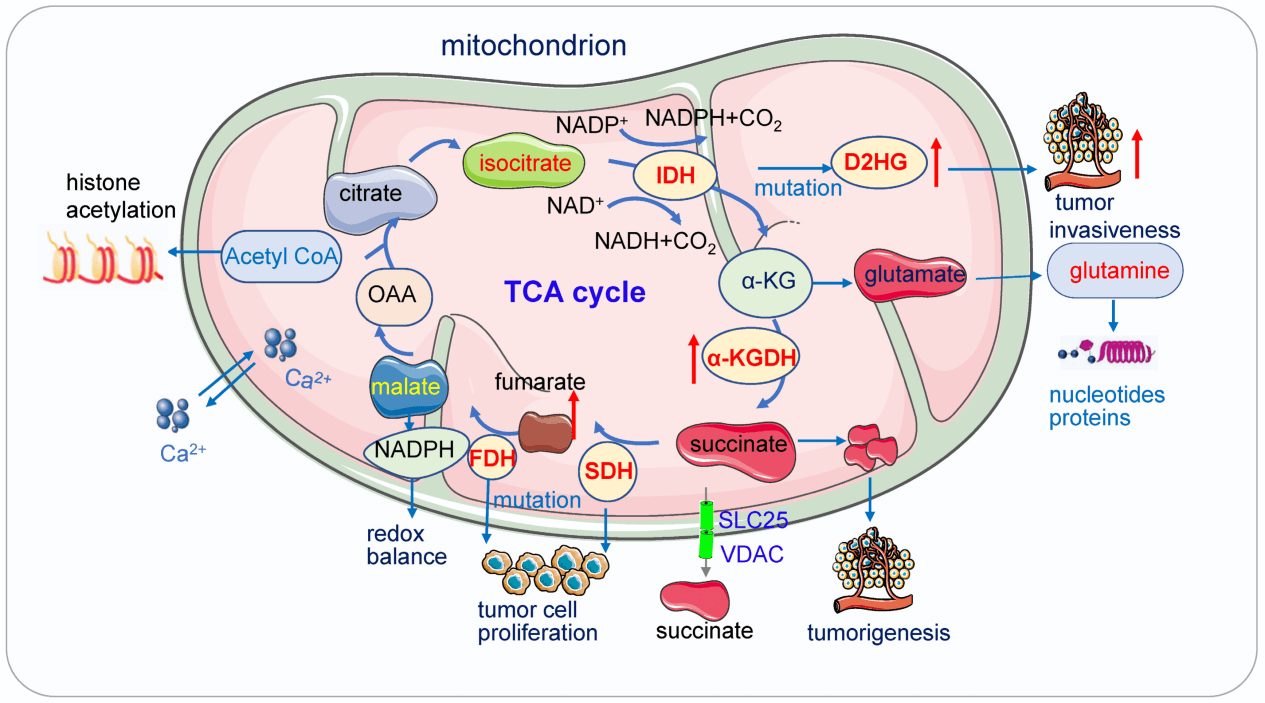
Fig. 2 Key alterations in the Mitochondrial TCA cycle associated with cancer[9].
03 Biomarkers of mitochondrial dysfunction in cancer
Reflecting the critical role of mitochondrial metabolism, biomarkers of mitochondrial dysfunction have emerged as valuable tools for early detection, prognostic stratification, and therapy monitoring. These biomarkers span genetic, metabolic, proteomic, and functional categories.
Genetic biomarkers: mutations in mitochondrial DNA (mtDNA), such as those affecting MT-CO1and MT-ND1, which encode subunits of oxidative phosphorylation complexes[10]. These mutations are associated with OXPHOS impairment and have been correlated with adverse outcomes in cancers including colorectal carcinoma. The accumulation of mtDNA variations, particularly in complexes I and III, may also influence reactive oxygen species (ROS) production and predict sensitivity to ROS-stimulating therapies.
Metabolic biomarkers: Elevated lactate level reflects warburg metabolism, while reduced ketone bodies indicate impaired mitochondrial β-oxidation[11]. Alterations in the arginine and citrulline ratio further signify dysregulated arginine metabolism, which supports tumor proliferation and immune evasion .
Protein biomarkers: Overexpression of FASN (implicated in fatty acid synthesis), ARG1 (involved in arginine metabolism), and p53 (a regulator of metabolic switching) highlights the interplay between oncogenic signaling and mitochondrial metabolic pathways. These proteins can indicate therapeutic vulnerabilities and disease aggressiveness[12].
Functional biomarkers: Reduced oxygen consumption rate (OCR), elevated ROS production, and loss of mitochondrial membrane potential (Δψm), provide dynamic measures of mitochondrial fitness[13]. Such parameters can be assessed via liquid biopsy or tissue-based assays, offering insights into real-time changes in cancer energy metabolism.
Integrating mitochondrial biomarkers with immunometabolic markers enhance the prediction of responses to immunotherapy[14], underscoring the value of multiparameter biomarker panels in metabolic oncology for personalizing treatment strategies. These biomarkers require robust functional assays to validate their clinical utility, which will be discussed in the following section.
04 Assays for mitochondrial function in cancer research
To validate the clinical relevance of mitochondrial biomarkers and dissect underlying metabolic perturbations, standardized functional assays are essential. Key assays for evaluating mitochondrial function in cancer research are summarized below.
Oxygen Consumption Rate (OCR): Using Seahorse XF technology or fluorescent probes, OCR quantifies mitochondrial respiration parameters (e.g., basal respiration, ATP-linked respiration, maximal respiratory capacity) in live cells.
Mitochondrial Membrane Potential (Δψm): Vital for ATP synthesis, Δψm loss indicates dysfunction. It is detectable with potentiometric dyes like JC-1 or TMRE.
Reactive Oxygen Species (ROS) Detection: Using probes like MitoSOX, this assay helps assess redox stress in mitochondria.
TCA Cycle Enzyme Activity Assays: Assays targeting enzymes such as citrate synthase serve as indicators of mitochondrial metabolic capacity and are useful for normalizing biochemical data.
ATP Production Assays: These assays distinguish between glycolytic and mitochondrial ATP sources, enabling determination of the primary energy pathway utilized by cancer cells.
Collectively, these assays provide a foundational toolkit for investigating how mitochondrial function influences cancer progression and therapy response.
05 Advanced techniques for analyzing mitochondrial metabolism in cancer cells
Recent technological advances have significantly enhanced the resolution and scope of mitochondrial metabolism analysis, providing powerful tools for metabolic oncology research.
Global metabolomics: Global metabolomics via mass spectrometry (MS) identifies mitochondrial metabolite signatures, such as TCA cycle intermediates and ketone bodies, in cancer cells and the tumor microenvironment (TME), facilitating studies on oncogenic metabolism[15].
Single-cell metabolic profiling: Single-cell metabolic profiling integrates flow cytometry with metabolic probes (e.g., pHrodo, MitoTracker), reveals functional heterogeneity in mitochondrial activity within tumor populations, enabling precise dissection of energy metabolism[16].
In Vivo Imaging Techniques: For in vivo assessment, imaging techniques like [18F] FDG PET-CT visualize glucose uptake, while mitochondrial-specific tracers (e.g., [18F] MISO) probe OXPHOS activity in preclinical and clinical models of lung cancer[17].
Functional Genomics and Proteomics: CRISPR-Cas9 high-throughput screening identifies key regulators of mitochondrial metabolism (e.g., genes essential for OXPHOS in IDH-mutant cancers) and synthetic lethal interactions[1]. Additionally, quantitative MS-based proteomics and lipidomics detect alterations in mitochondrial enzyme expression (e.g., FASN, SDH) and lipid composition, linking mitochondrial function to therapy resistance in fatty acid-dependent cancers[1].
Collectively, these methods have uncovered metabolic codependencies within the TME and validated mitochondrial remodeling as a critical driver of cancer progression, such as in lung cancer metastasis.
06 Therapeutic strategies targeting mitochondrial dysfunction in cancer
Targeting mitochondrial metabolism represents a growing frontier in metabolic oncology, with multiple therapeutic strategies showing promise in preclinical and clinical settings for cancers reliant on specific metabolic alterations.
Mitochondrial Enzyme Inhibitors
Inhibitors targeting key mitochondrial-associated enzymes are under investigation. For instance, FASN inhibitors (e.g., TVB-3166) impede de novofatty acid synthesis, disrupting energy production and biosynthetic processes in cancer cells, which can lead to tumor regression[1].
Arginine Deprivation Therapy
This approach exploits the metabolic vulnerability of arginine auxotrophic cancers. Administering agents like PEGylated arginase (PEG-Arg) depletes extracellular arginine, selectively targeting cancer cells with deficiencies in arginine synthesis, such as hepatocellular carcinoma[18] .
Ketogenic Metabolic Therapy (KMT)
KMT utilizes a low-carbohydrate, high-fat dietto induce a state of ketosis. This metabolic shift can impair the growth of some tumors by reducing the availability of glucose, potentially sensitizing cancers to conventional chemotherapy and immunotherapy[19] .
OXPHOS Inhibitors
Compounds like IACS-010759, which inhibit mitochondrial complex I, directly target OXPHOS. This strategy is particularly relevant for cancers dependent on mitochondrial respiration, such as certain subtypes of non-small cell lung cancer[20].
Rational Combination Therapies
Combining mitochondrial-targeted agents with other treatments enhances efficacy. For example, FASN inhibitors can synergize with immune checkpoint blockers to counteract immunosuppressive microenvironments. Similarly, KMT is explored alongside therapies like CAR-T cells to improve their anti-tumor function by modulating T cell metabolism.
Recommended Elabscience® Mitochondrial Function Assay Kits
Table 1. Assay Kits for Mitochondrial Functional Research
|
Cat. No. |
Product Name |
|
E-BC-F064 |
Mitochondrial Permeability Transition Pore (mPTP) Fluorometric Assay Kit |
|
E-BC-F070 |
Enhanced Oxygen Consumption Rate (OCR) Fluorometric Assay Kit |
|
E-BC-F300 |
ATP Assay Kit |
|
E-CK-A301 |
Mitochondrial Membrane Potential Assay Kit (with JC-1) |
|
E-BC-F078 |
Mitochondrial Stress Fluorometric Assay Kit |
|
E-BC-K784-M |
Fatty Acid Oxidation (FAO) Colorimetric Assay Kit |
|
E-BC-K138-F |
Reactive Oxygen Species (ROS) Fluorometric Assay Kit (Green) |
|
E-BC-K649-M |
Succinate Dehydrogenase (SDH) Activity Assay Kit |
|
E-BC-K651-M |
NAD-Isocitrate Dehydrogenase (NAD-IDH) Activity Assay Kit |
|
E-BC-K785-M |
β-Hydroxybutyrate (Ketone Body) Colorimetric Assay Kit |
|
E-BC-F008 |
Mitochondrial Superoxide Fluorometric Assay Kit |
|
E-BC-F005 |
Reactive Oxygen Species (ROS) Fluorometric Assay Kit (Red) |
References:
[1] Du H, Xu T, Yu S, Wu S, Zhang J. Mitochondrial metabolism and cancer therapeutic innovation. Signal Transduct Target Ther. 2025;10(1):245. doi:10.1038/s41392-025-02311-x
[2]Gao Y, Xiong Z, Wei X. Mitochondrial Metabolomics in Cancer: Mass Spectrometry-Based Approaches for Metabolic Rewiring An alysisand Therapeutic Discovery. Metabolites. 2025;15(8):513. doi:10.3390/metabo15080513
[3] Arismendi-Morillo, G., Duraj, T., Lee, D., Mukherjee, P. & Seyfried, T. (). From mitochondrial cristae pathobiology to metabolic reprogramming in cancer: the α and ω of Malignancies?. Oncologie. /doi.org/10.1515/oncologie-2025-0379
[4] Clay R, Li K, Jin L. Metabolic Signaling in the Tumor Microenvironment. Cancers (Basel). 2025;17(1):155. doi:10.3390/cancers17010155
[5] Ren L, Liu W, Zheng J, Wu Q, Ai Z. The Role of Mitochondrial Genome Stability and Metabolic Plasticity in Thyroid Cancer. Biomedicines. 2025; 13(11):2599. doi.org/10.3390/biomedicines13112599
[6] Missiroli S, Perrone M, Genovese I, Pinton P, Giorgi C. Cancer metabolism and mitochondria: Finding novel mechanisms to fight tumours. EBioMedicine. 2020;59:102943. doi:10.1016/j.ebiom.2020.102943
[7] Eltayeb K, La Monica S, Tiseo M, Alfieri R, Fumarola C. Reprogramming of Lipid Metabolism in Lung Cancer: An Overview with Focus on EGFR-Mutated Non-Small Cell Lung Cancer. Cells. 2022;11(3):413. doi:10.3390/cells11030413
[8] Chen CL, Hsu SC, Ann DK, Yen Y, Kung HJ. Arginine Signaling and Cancer Metabolism. Cancers (Basel). 2021;13(14):3541. doi:10.3390/cancers13143541
[9] Liu Y, Sun Y, Guo Y, et al. An Overview: The Diversified Role of Mitochondria in Cancer Metabolism. Int J Biol Sci. 2023;19(3):897-915. doi:10.7150/ijbs.81609
[10] Lei T, Rui Y, Xiaoshuang Z, Jinglan Z, Jihong Z. Mitochondria transcription and cancer. Cell Death Discov. 2024;10(1):168. doi:10.1038/s41420-024-01926-3
[11] Morelli AM, Scholkmann F. Should the standard model of cellular energy metabolism be reconsidered? Possible coupling between the pentose phosphate pathway, glycolysis and extra-mitochondrial oxidative phosphorylation. Biochimie. 2024;221:99-109. doi:10.1016/j.biochi.2024.01.018
[12] Zhang L, Wei Y, Yuan S, Sun L. Targeting Mitochondrial Metabolic Reprogramming as a Potential Approach for Cancer Therapy. Int J Mol Sci. 2023;24(5):4954. Published 2023 Mar 4. doi:10.3390/ijms24054954
[13] Park WH. The mitochondrial nexus: Targeting metabolic vulnerabilities, oxidative stress, and immunomodulation to induce cancer cell death. Biochim Biophys Acta Rev Cancer. Published online October 31, 2025. doi:10.1016/j.bbcan.2025.189491
[14]Cui X, Liu X, Feng R, et al. Editorial: Tumor microenvironment and metabolic reprogramming in cancer. Front Immunol. 2024;15:1497966. doi:10.3389/fimmu.2024.1497966
[15] Gao Y, Xiong Z, Wei X. Mitochondrial Metabolomics in Cancer: Mass Spectrometry-Based Approaches for Metabolic Rewiring Analysis and Therapeutic Discovery. Metabolites. 2025;15(8):513. Published 2025 Jul 31. doi:10.3390/metabo15080513
[16] Mao X, Xia D, Xu M, et al. Single-Cell Simultaneous Metabolome and Transcriptome Profiling Revealing Metabolite-Gene Correlation Network. Adv Sci (Weinh). 2025;12(4):e2411276. doi:10.1002/advs.202411276
[17] Monaco L, Crivellaro C, De Bernardi E, et al. Next-generation radiomic sequencing in non-small cell lung cancer: an alternative model to predict mutations from [18F]FDG PET/CT. Ther Adv Respir Dis. 2025;19:17534666251384433. doi:10.1177/17534666251384433
[18] Saha P, Ettel P, Weichhart T. Leveraging macrophage metabolism for anticancer therapy: opportunities and pitfalls. Trends Pharmacol Sci. 2024;45(4):335-349. doi:10.1016/j.tips.2024.02.005
[19] Weber DD, Aminazdeh-Gohari S, Kofler B. Ketogenic diet in cancer therapy. Aging (Albany NY). 2018;10(2):164-165. doi:10.18632/aging.101382
[20] Zhou Y, Zou J, Xu J, Zhou Y, Cen X, Zhao Y. Recent advances of mitochondrial complex I inhibitors for cancer therapy: Current status and future perspectives. Eur J Med Chem. 2023;251:115219. doi:10.1016/j.ejmech.2023.115219



