Enzyme-linked immunosorbent assay (ELISA) is a ubiquitous immunological technique extensively utilized in basic scientific research, clinical translational studies, and diagnostic practice. The core principle of this assay rests on the specific interaction between a target antigen (i.e., the protein of interest) and its cognate primary antibody. Antigen presence is then validated via the catalytic activity of enzyme-conjugated antibodies toward an exogenously added substrate; the resulting reaction products can be analyzed either qualitatively by visual inspection or quantitatively through signal readouts obtained using a luminometer or spectrophotometer. Based on variations in antigen-antibody configurations, substrate selection, and experimental parameters, ELISA methodologies are broadly categorized into four major formats: direct, indirect, sandwich, and competitive ELISA[1].
This article reviews the historical evolution of ELISA, methodological advancements driven by market demands, diverse strategies for one-step ELISA implementation, and optimization approaches for one-step ELISA detection.
Table of Contents
1. The Historical Development of ELISA
2. Principles Underlying the simple step ELISA
3. Sensitivity Optimization Strategies for the simple step ELISA Assay
01 The Historical Development of ELISA
The development of the ELISA,now a ubiquitous immunological detection technique,traces back to the 1960s, when two independent research groups concurrently conceptualized ELISA and enzyme immunoassay (EIA) methodologies through the modification of radioimmunoassay (RIA) protocols. These pioneering teams included Eva Engvall and Peter Perlmann at Stockholm University in Sweden, and Anton Schuurs and Bauke van Weemen in the Netherlands. Fundamentally, ELISA capitalizes on the inherent specificity of antigen-antibody interactions(a hallmark of the adaptive immune response)with target antigens detected via enzyme-conjugated antibody probes. Notably, Engvall and Perlmann first formalized the ELISA technique in the 1970s by conjugating target proteins to the enzyme alkaline phosphatase (AP)[2,3]. Concurrently, van Weemen and Schuurs reported a analogous immunoassay platform, which instead employed horseradish peroxidase (HRP) as the reporter enzyme[2,3]. The core technical framework of ELISA thus stemmed from RIA optimization, wherein the radioactive iodine-125 tracer used in RIA was substituted with stable enzymatic labels (i.e., AP and HRP)[1,2].Over the following decade, a diverse array of ELISA derivative formats was rapidly developed and standardized. At the methodological level, variants including indirect, sandwich, and competitive ELISA were introduced; these iterations not only enhanced assay sensitivity and specificity but also enabled the detection of low-molecular-weight haptens. In parallel, advancements in solid-phase carriers,most notably the transition from test tubes to 96-well polystyrene microplates,paved the way for high-throughput analysis, automated operation, and rigorous standardization of immunoassay workflows.
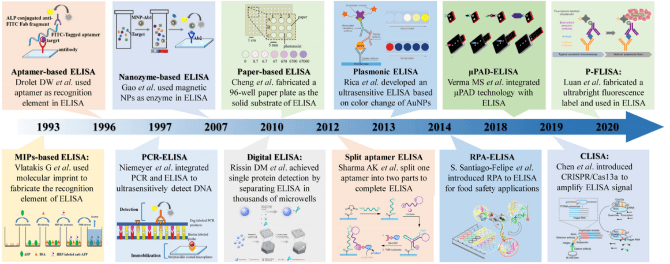
Fig. 1 The evolution overview of representative ELISA derived technologies in recent decades[4].
02 Principles Underlying the simple step ELISA
There are different types of ELISA techniques used depending on the specific needs of the researcher or the clinician. These are direct, indirect, sandwich, and competitive ELISA, which vary based on the types of antibodies, antigens, substrates, and experimental conditions used[1].Of these methodologies, sandwich and competitive ELISA are the most extensively implemented in practical applications. Sandwich ELISA principle and ELISA workflow underpin its technical utility: the core advantages of sandwich ELISA over alternative formats include diminished background signal, superior sensitivity, and robust applicability for quantifying antigens in complex biological matrices[5]. By contrast, competitive ELISA is particularly valued for its compatibility with small-molecule analytes lacking multiple epitopes, heightened specificity conferred by competitive antigen-antibody binding dynamics, streamlined workflows that minimize antibody usage, and exceptional resilience to matrix interference in complex biological samples. As research across diverse biomedical disciplines advances, the demand for high-efficiency immunoassay platforms has intensified progressively. To address this imperative, ELISA technology has undergone transformative innovation: transitioning from traditional three-step ELISA assay protocol involving sequential incubation and washing steps(typically requiring 3-4 hours)to modern one-step formats that complete the entire assay in only 1.5 hours. This evolution has substantially enhanced experimental efficiency and operational convenience, with ELISA procedure steps simplified to facilitate high-throughput sample processing in routine laboratory settings. Currently, a broad spectrum of one-step ELISA kits is commercially available, and a comprehensive overview of these products is presented in the subsequent section.
One-step sandwich method: Conventional
The capture antibody is pre-immobilized on the surface of microplate wells. For the assay, samples or standard solutions are co-incubated with HRP-conjugated detection antibodies, facilitating the formation of a sandwich immunocomplex consisting of capture antibody–antigen–enzyme-linked detection antibody. Following thorough washing to remove unbound components, a chromogenic substrate solution is introduced to initiate the enzymatic reaction.
One-step sandwich method: Tag antibodies
Through genetic engineering approaches, a tag is fused to the Fc region of the capture antibody, while an anti-tag antibody is pre-immobilized on the microplate wells. At assay initiation, standard solutions or samples are co-administered with an antibody cocktail(comprising the Fc-tagged capture antibody and HRP-conjugated detection antibody)enabling one-step formation of a sandwich immunocomplex with the configuration: anti-tag antibody–tagged capture antibody-target antigen-HRP-conjugated detection antibody.
One-step sandwich method: Microspheres
A target-specific capture antibody was pre-immobilized on high-affinity microplate wells, with HRP microspheres and detection antibody microspheres pre-deposited in each well. Upon addition of standard solutions and test samples to the microplate wells, a sandwich immunocomplex (capture antibody–target antigen–detection antibody–HRP) is formed.
One-step competition method: Conventional
The target antigen is pre-immobilized onto the wells of a 96-well microplate. For the assay, test samples or standard solutions are co-incubated with HRP-conjugated antibodies. The antigen present in the samples or standards competes with the plate-immobilized antigen for binding to the HRP-conjugated antibodies, leading to the formation of immobilized antigen-HRP-conjugated antibody complexes.
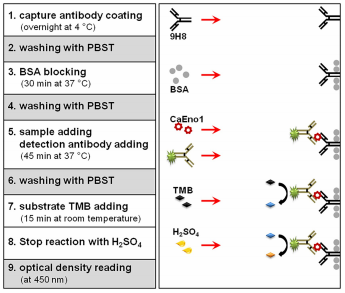
Fig. 2 Schematic representation of the one-step double monoclonal antibody sandwich ELISA assay[6].
03 Sensitivity Optimization Strategies for the simple step ELISA Assay
One step ELISA kits, a representative form of rapid elisa, outperform their traditional counterparts substantially in terms of assay turnaround time and operational simplicity; however, they do not exhibit a distinct advantage in analytical sensitivity. For certain target analytes, the sensitivity of one-step formats may even be inferior to that of conventional multi-step elisa protocol. Consequently, optimizing the sensitivity of one-step ELISA has emerged as a critical challenge for immunoassay development, especially considering the growing demands of elisa applications. Based on the chromogenic reaction principle of ELISA, enhancing analytical sensitivity requires maximizing the interaction between HRP and 3,3',5,5'-tetramethylbenzidine (TMB) substrates. This objective has driven the exploration of two primary technical strategies: first, augmenting the loading capacity of HRP molecules such that multiple HRP moieties can be conjugated to a single detection antibody within the immunocomplex responsible for chromogenic signal generation; second, engineering tandem arrays of HRP molecules for subsequent conjugation to detection antibodies, thereby achieving the presentation of multiple serially linked HRP units on a single immunocomplex.
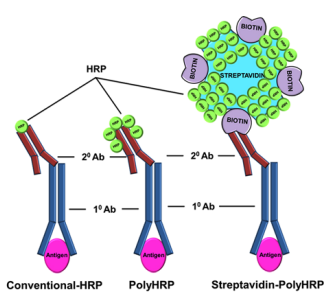
Fig. 3 Polymeric HRP[7].
In summary, ELISA assay is a core technology. It developed based on radioimmunoassay in the 1970s. The emergence of forms such as sandwich method and competitive method has enhanced its performance. To meet the demand for efficient detection, the one-step method emerged. By mixing the sample with the detection reagent in one go, it significantly shortens the process time. Current development focuses on optimizing its sensitivity by enhancing signal amplification, providing support for fast and highly sensitive detection applications.
Elabscience® QuicKey Pro of Popular Products
Table 1. Research Tools for cytokines
|
Cat. No. |
Product Name |
|
E-OSEL-H0001 |
QuicKey Pro Human IL-6(Interleukin 6) ELISA Kit |
|
E-OSEL-H0006 |
QuicKey Pro Human Cortisol ELISA Kit |
|
E-OSEL-H0014 |
QuicKey Pro Human IL-8(Interleukin 8) ELISA Kit |
|
E-OSEL-H0036 |
QuicKey Pro Human GDF15 (Growth Differentiation Factor 15) ELISA Kit |
|
E-OSEL-H0049 |
QuicKey Pro Human VEGFR-2 (Vascular Endothelial Growth Factor Receptor 2) ELISA Kit |
|
E-OSEL-H0060 |
QuicKey Pro Human IL-6R (Interleukin 6 Receptor) ELISA Kit |
|
E-OSEL-H0067 |
|
|
E-ELIR-020 |
PolyHRP-Streptavidin |
References:
[1] Hayrapetyan, H., Tran, T., Tellez-Corrales, E., & Madiraju, C. (2023). Enzyme-linked immunosorbent assay: types and applications.ELISA: methods and protocols, 1-17.
[2] Engvall E, Perlmann P (1971) Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8(9):871–874
[3] Alhajj M, Farhana A (2022) Enzyme linked immunosorbent assay. In StatPearls, Treasure Island (FL)
[4] Peng, P., Liu, C., Li, Z., Xue, Z., Mao, P., Hu, J., ... & You, M. (2022). Emerging ELISA derived technologies for in vitro diagnostics.TrAC Trends in Analytical Chemistry,152, 116605.
[5] Tabatabaei, M. S., & Ahmed, M. (2022). Enzyme-linked immunosorbent assay (ELISA). In Cancer cell biology: Methods and protocols(pp. 115-134). New York, NY: Springer US.
[6] Piao, J., Li, N., Zhang, L., Meng, H., Sun, Q., & He, Z. (2023). Quantitatively detecting Candida albicans enolase1 with a one-step double monoclonal antibody sandwich ELISA assay. Frontiers in Microbiology,14, 1078709.
[7] Mishra, M., Tiwari, S., Gunaseelan, A., Li, D., Hammock, B. D., & Gomes, A. V. (2019). Improving the sensitivity of traditional Western blotting via Streptavidin containing Poly‐horseradish peroxidase (PolyHRP).Electrophoresis,40(12-13), 1731-1739.



