Cytotoxic T cells (CTLs) represent a cornerstone of the immune system’s capacity to combat cancer, viral infections, and other aberrant cellular disorders. They are defined by their ability to specifically recognize and eliminate target cells via precise molecular mechanisms. Within the realm of cancer immunotherapy, their activation pathways and functional mechanisms have been extensively investigated and harnessed to improve therapeutic efficacy.
This review presents a comprehensive overview of their functions, core mechanisms, activation processes, and pivotal roles in cancer immunotherapy.
Table of Contents
1. What are cytotoxic T cells?
2. Cytotoxic T cell activation process
3. Function of cytotoxic T lymphocytes
4. Cytotoxic T cell markers
5. Mechanisms of cytotoxic T cell-mediated killing
6. Cytotoxic T cells and cancer immunotherapy
01 What are cytotoxic T cells?
Cytotoxic T cells, also known as CD8+ T cells, are a specialized subset of T lymphocytes within the adaptive immune system that play a crucial role in cell-mediated immunity. Their primary function is to identify and eliminate infected, cancerous, or otherwise abnormal cells through direct interactions.
Cytotoxic T cells originate from hematopoietic stem cells in the bone marrow and migrate to the thymus, where they undergo several stages of differentiation[1]:
Progenitor Stage: Bone marrow-derived progenitor cells migrate to the thymus as thymocytes.
Double-Negative (DN) Stage: In the initial stage of development, thymocytes lack both CD4 and CD8 surface markers (CD4-CD8-). During this phase, the rearrangement of the T-cell receptor (TCR) genes begins[1].
Double-Positive (DP) Stage: Once the TCR β-chain successfully rearranges and pairs with a surrogate α-chain, thymocytes express both CD4 and CD8 receptors (CD4+CD8+). At this stage, TCR α-chain rearrangement occurs[1].
Positive Selection: Thymocytes with TCRs that recognize self-antigens presented by Major Histocompatibility Complex Class I (MHC I) molecules on cortical epithelial cells are selected to differentiate into CD8+ T cells. Those recognizing MHC Class II develop into CD4+ T-helper cells[1].
Negative Selection: T cells that strongly bind self-antigens are eliminated to ensure self-tolerance. This process prevents autoimmunity.
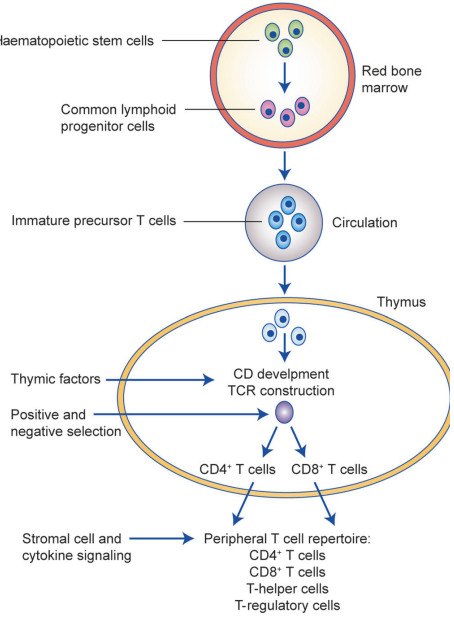
Fig .1 T-cell differentiation[1].
The surviving thymocytes leave the thymus as naive CD8+ cytotoxic T cells, expressing a TCR specific for foreign peptides presented by MHC I molecules.
02 Cytotoxic T cell activation process
The activation of cytotoxic T cells (typically CD8+ T cells) represents a sophisticated immunological event involving intricate interactions and multi-layered regulation between innate and adaptive immunity. Below is an authoritative and comprehensive dissection of their activation process:
2.1 Antigen Recognition and Initial Activation
Antigen Recognition: Cytotoxic T cells recognize processed antigenic peptides presented by antigen-presenting cells (APCs) via the T cell receptor (TCR). These peptides bind to major histocompatibility complex (MHC) class I molecules and are displayed on the APC surface. Dendritic cells (DCs) are among the most potent APCs, capable of presenting exogenous antigens to CD8+ T cells through cross-presentation mechanisms[2].
Costimulation: The activation of cytotoxic T cells requires costimulatory signals. The most prevalent costimulation is mediated by the binding of CD28 to CD80/CD86 on APCs. Additionally, interactions between other molecules (e.g., CD27 and CD70) serve as critical pathways to enhance activation signals, promoting T cell expansion and survival[3].
Cytokine Signaling: Cytokines including IL-2, IL-12, and type I interferons are essential for sustaining early activation. IL-2 acts as both an autocrine and paracrine factor, being particularly critical for the proliferation of CD8+ T cells[2,4].
2.2 Clonal Expansion and Effector Phase
Clonal Expansion: Activated CD8+ T cells enter a phase of rapid division, generating numerous clones to eliminate pathogens. This process typically involves up to seven rounds of division, leading to massive accumulation of effector cells[4]. Proliferation is accompanied by metabolic remodeling, with preferential activation of glycolysis and mitochondrial respiration to meet high energy demands.
Acquisition of Effector Functions: Upon activation, CD8+ T cells acquire effector functions, including secretion of cytokines (e.g., IFN-γ, TNF-α) and release of cytolytic molecules (e.g., perforin and granzymes), to eliminate infected or malignant target cells[4].
2.3 Differentiation into Memory CD8+ T Cells
Regulation After Antigen Clearance: Following pathogen elimination, most effector cells undergo apoptosis, while a small subset differentiates into memory CD8+ T cells. The transcription factors T-bet and Eomesodermin (Eomes) are core regulators of the effector-to-memory transition[4].
Central memory T cells (TCM): Migrate to lymphoid tissues via CCR7 and CD62L, exhibiting long-term persistence and high proliferative capacity.
Effector memory T cells (TEM): Localize to peripheral tissues and display rapid effector responses.
Tissue-resident memory T cells (TRM): Reside permanently in barrier tissues, providing local protection[4,5].
Mechanisms of Long-Term Maintenance: IL-7 and IL-15 play pivotal roles in the survival and renewal of memory CD8+ T cells; these cytokines sustain the homeostasis of gene expression and cellular metabolism[4,5]. Memory cells exhibit a metabolic preference for fatty acid oxidation and mitochondrial biogenesis, contrasting with effector cells that prioritize rapid energy supply via glycolysis.
03 Function of cytotoxic T lymphocytes
As a central mediator of adaptive immunity, CD8+ T cells play a pivotal, multifaceted role in host defense by directly eliminating infected or malignant cells and orchestrating broader immune responses. They execute specific cytotoxicity through the release of perforin-granzyme complexes and Fas-FasL signaling, which induce targeted apoptosis, while concurrently secreting cytokines (e.g., IFN-γ, TNF-α) to amplify antigen presentation and activate other immune effectors[13,14]. Endowed with antigen specificity via T cell receptor (TCR) recognition of peptide-major histocompatibility complex class I (peptide-MHC I) complexes, CD8+ T cells ensure precise target recognition. Following pathogen clearance, a subset of activated CD8+ T cells differentiates into long-lived memory populations (encompassing central, effector, and tissue-resident memory subsets) to deliver rapid, durable protection upon re-exposure[4,5]. Furthermore, CD8+ T cells sustain immune homeostasis through regulatory circuits such as the PD-1/PD-L1 checkpoint[11,12]. These integrated functional properties underscore their indispensable role in controlling viral infections and tumors, and establish the mechanistic foundation for emerging immunotherapies, such as CAR-T cell therapy and cancer vaccines[11,12].
04 Cytotoxic T cell markers
Cytotoxic T cells (Cytotoxic CD8+ T Cells, CTLs; also referred to as CD8+ T cells) are primarily identified and distinguished by specific surface markers. These molecules not only play crucial roles in T cell classification but are also closely associated with T cell activation status, functional differentiation, and memory formation. Below is a comprehensive analysis of CTL markers.
Table. 1 Summary Table of Cytotoxic T Cell Markers[7,8,9,21]
|
Category |
Marker |
Primary Function |
Significance and Characteristics |
|
Core Defining Markers |
CD8 |
Co-receptor for MHC class I molecules; stabilizes TCR-antigen engagement. |
The definitive surface marker for identifying cytotoxic T lymphocytes (CTLs), conventionally termed CD8+ T cells. |
|
TCR (T Cell Receptor) |
Recognizes specific antigenic peptides presented by MHC class I molecules. |
The key receptor responsible for antigen-specific target cell recognition and killing. |
|
|
Functional & Cytotoxic Markers |
Perforin |
Pore-forming protein that facilitates the entry of apoptotic agents into the target cell. |
A key indicator of cytotoxic potential; stored in cytoplasmic granules. Detection typically requires intracellular staining. |
|
Granzyme B |
Serine protease that initiates caspase-dependent and -independent apoptotic pathways upon entry into the target cell. |
A canonical effector molecule and a classic marker for activated, cytotoxic CD8+ T cells. Requires intracellular staining for detection. |
|
|
Granulysin |
Exhibits antimicrobial activity and can induce apoptosis in target cells. |
Contributes to the elimination of pathogen-infected cells and tumors. |
|
|
Activation, Proliferation & Memory Markers |
CD69 |
A type II transmembrane glycoprotein; functions as an early activation antigen. |
Used to identify recently activated T cells. |
|
CD25 (IL-2Rα) |
Alpha chain of the high-affinity interleukin-2 (IL-2) receptor. |
Upregulated upon T cell activation, enabling robust proliferative responses to IL-2. Also expressed on Tregs, requiring careful gating. |
|
|
CD62L (L-Selectin) |
A homing receptor that mediates lymphocyte entry into secondary lymphoid organs. |
Critical for delineating memory T cell subsets: Central Memory T cells (TCM) are CD62L+, while Effector Memory T cells (TEM) are typically CD62L-. |
|
|
CD45 Isoforms |
Protein tyrosine phosphatase essential for TCR signaling. |
Isoform switching occurs upon activation: Naive T cells express CD45RA, whereas antigen-experienced effector and memory T cells express CD45RO (in humans). |
|
|
CD127 (IL-7Rα) |
Alpha chain of the interleukin-7 (IL-7) receptor. |
High expression is associated with memory precursor cells and long-lived memory T cells; often downregulated in short-lived effector cells. |
|
|
Inhibitory & Exhaustion Markers |
PD-1 (Programmed Death-1) |
An immunoinhibitory receptor that attenuates T cell responses upon binding to its ligands (PD-L1/PD-L2). |
The prototypical marker of T cell exhaustion. Sustained high expression correlates with progressive dysfunction and is a primary target for checkpoint blockade therapy. |
|
Tim-3 |
Co-inhibitory receptor involved in immune tolerance and exhaustion. |
Frequently co-expressed with PD-1 on severely exhausted T cells; a target for next-generation immunotherapies. |
|
|
LAG-3 |
Co-inhibitory receptor that negatively regulates T cell proliferation and function. |
Often co-expressed with PD-1; a major target for combinatorial immunotherapy approaches. |
Table. 2 Representative Flow Cytometry Panels for Cytotoxic T Cell Subsets[8,10,21]
|
T Cell Subset |
Canonical Surface and Intracellular Marker Phenotype |
|
Naive T Cells |
CD3+ CD8+ CD45RA+ CD62L+ CD27+ CCR7+ |
|
Effector T Cells |
CD3+ CD8+ CD45RA+ Granzyme B+ Perforin+ |
|
Central Memory T Cells (TCM) |
CD3+ CD8+ CD45RO+ CD62L+ CD127+ |
|
Effector Memory T Cells (TEM) |
CD3+ CD8+ CD45RO+ CD62L- |
|
Exhausted T Cells |
CD3+ CD8+ PD-1hi Tim-3+ LAG-3+ (Often accompanied by reduced expression of Granzyme B and Perforin) |
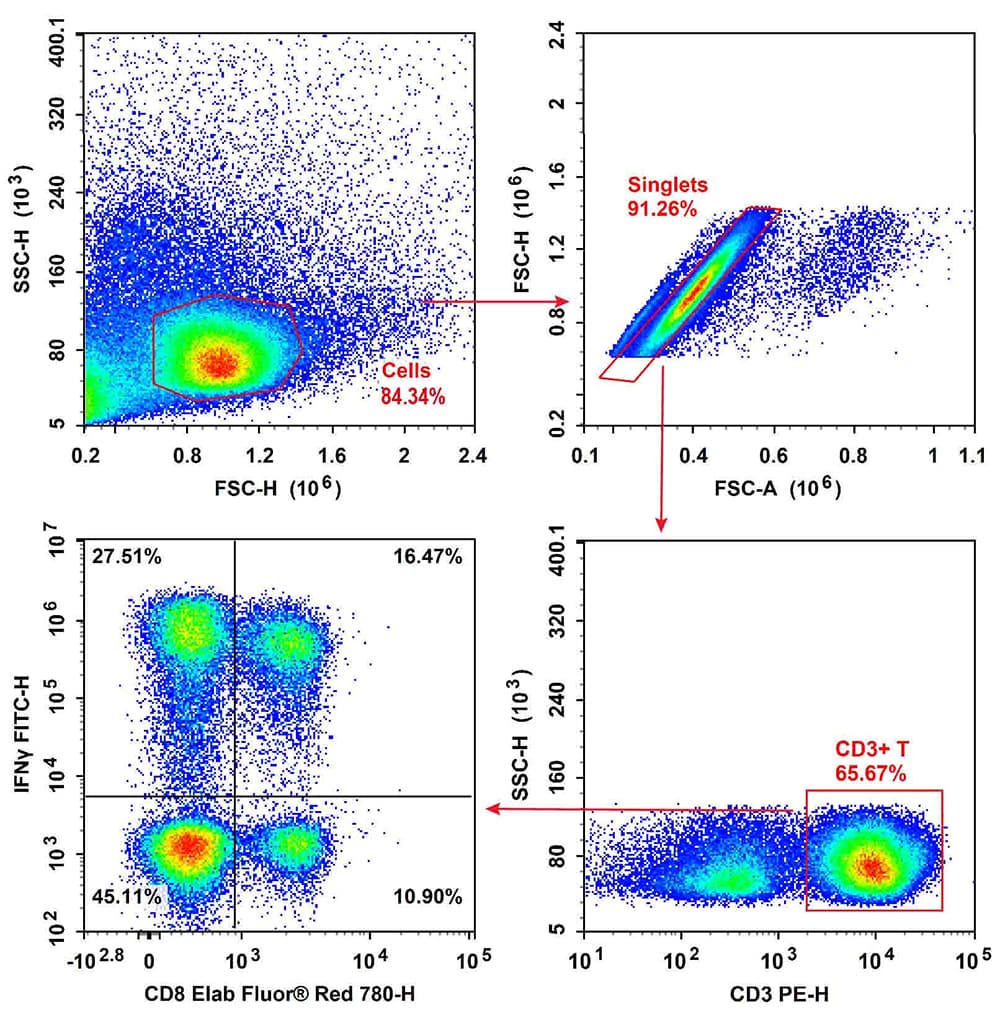
Fig. 2 Normal human peripheral blood cells were stimulated with Cell Stimulation MIX and Protein Transport Inhibitor MIX. Then PBMCs were fixed, permeabilized, and stained with PE Anti-Human CD3, Elab Fluor® Red 780 Anti-Human CD8a, and FITC Anti-Human IFN-γ, followed by analysis via flow cytometry. Tc1 cells exhibited the phenotype CD3+CD8+IFN-γ+, Tc2 cells exhibited the phenotype CD3+CD8+IFN-γ-.
Elabscience® Quick Overview of Popular Products:
Table. 3 Research Tools for Cytotoxic T Cells
|
Product Name |
Cat. No. |
|
PE Anti-Human CD3 Antibody[OKT-3] |
E-AB-F1001D |
|
Elab Fluor® Red 780 Anti-Human CD8a Antibody[OKT-8] |
E-AB-F1110S |
|
FITC Anti-Human IFN-γ Antibody[B27] |
E-AB-F1196C |
|
PE/Cyanine7 Anti-Human CD45RO Antibody[UCHL1] |
E-AB-F1139H |
|
PE/Cyanine5.5 Anti-Human CD45RA Antibody[HI100] |
E-AB-F1052I |
|
PE Anti-Human CD69 Antibody[FN50] |
E-AB-F1138D |
|
PerCP/Cyanine5.5 Anti-Human CD127/IL-7RA Antibody[A019D5] |
E-AB-F1152J |
|
Elab Fluor® 700 Anti-Human CD279/PD-1 Antibody[EH12.2H7] |
E-AB-F1229M1 |
|
APC Anti-Human CD25 Antibody[BC96] |
E-AB-F1194E |
|
FITC Anti-Human CD197/CCR7 Antibody[G043H7] |
E-AB-F1159C |
|
Elab Fluor® 700 Anti-Human CD194/CCR4 Antibody[L291H4] |
E-AB-F1366M1 |
|
Human PBMC Separation Solution(P 1.077) |
E-CK-A103 |
|
EasySort™ Human Naïve Pan T Cell Isolation Kit |
MIH006N |
|
EasySort™ Human Naïve CD8+T Cell Isolation Kit |
MIH008N |
|
EasySort™ Mouse Naïve CD8+T Cell Isolation Kit |
MIM008N |
|
Cell Stimulation and Protein Transport Inhibitor Kit |
E-CK-A091 |
|
Human CD3/CD28 T Cell Activation Beads |
MIH001A |
|
CFSE Cell Division Tracker Kit |
E-CK-A345 |
|
EasySort™ Human CD3+T Cell Isolation Kit |
MIH001N |
|
Human IFN-γ (Interferon Gamma) ELISPOT Kit |
ESP-H0002 |
|
Human TNF-α (Tumor Necrosis Factor Alpha) ELISPOT Kit |
ESP-H0010 |
|
Human IL-10 (Interleukin 10) ELISPOT Kit |
ESP-H0003 |
|
Human IL-4 (Interleukin 4) ELISPOT Kit |
ESP-H0007 |
|
Human IL-17A (Interleukin 17A) ELISPOT Kit |
ESP-H0004 |
|
Mini Sample Human GzmB ( Granzyme B ) ELISA Kit |
E-MSEL-H0019 |
|
Human GzmB(Granzyme B) ELISA Kit |
E-EL-H1617 |
|
Human PRF1(Perforin 1) ELISA Kit |
E-EL-H1123 |
05 Mechanisms of cytotoxic T cell-mediated killing
CD8+ T cell-mediated cytotoxicity is primarily executed through the following pathways:
Granule-Dependent Cytotoxic Pathway: Through the granule-dependent cytotoxic pathway, activated CD8+ T lymphocytes mediate target cell killing by releasing specialized cytoplasmic granules containing perforin and granzymes. Perforin facilitates the delivery of granzymes into the target cell cytoplasm by forming pores in the target cell membrane. Once internalized, granzymes activate caspase-dependent apoptotic pathways, leading to systematic DNA fragmentation and ultimately, programmed cell death[14].
Fas-FasL Pathway: The Fas-FasL pathway operates through the binding of Fas ligand (FasL) expressed on CD8+ T cells to the Fas receptor on the target cell surface, which initiates a signaling cascade leading to the activation of Caspase-8. Subsequently, Caspase-8 activates Caspase-3 via downstream cascades, ultimately inducing apoptosis of the target cell[13].
Cytokine-Assisted Cytotoxicity: CD8+ T cells execute their cytotoxic function not only through direct cell-to-cell contact but also via the secretion of key effector cytokines, notably Interferon-γ (IFN-γ) and Tumor Necrosis Factor-α (TNF-α). IFN-γ enhances the immunogenicity of target cells by upregulating their MHC-I expression and concurrently activates macrophages to bolster broader anti-pathogen immunity. In parallel, TNF-α contributes to target cell elimination by directly inducing apoptotic signaling and exerts pleiotropic effects that modulate the overall immune microenvironment[15].
Characteristic Mechanisms of Target Cell Death: Upon recognition and engagement by CD8+ T cells, target cells undergo characteristic morphological and biochemical changes indicative of programmed cell death. Key hallmarks include the initiation of DNA fragmentation, driven primarily by the synergistic action of granzymes and activated caspases. This is accompanied by classic apoptotic markers such as phosphatidylserine externalization, detectable by Annexin V staining. Furthermore, critical intracellular metabolic disruptions occur, notably mitochondrial dysfunction, which precipitates a loss of membrane potential and amplifies the apoptotic cascade[16].
Regulation and Pathological States: CD8+ T cell-mediated cytotoxicity is essential for the clearance of pathogens and tumors; however, its dysregulation can lead to significant immunopathology. In the tumor microenvironment, cancer cells frequently upregulate PD-L1, which engages PD-1 on T cells to suppress effector functions, a form of immune evasion reversible by checkpoint blockade. Under conditions of chronic antigen exposure, such as persistent viral infection or cancer, CD8+ T cells may develop an exhausted state, marked by progressive loss of cytolytic activity and altered expression of transcription factors like TOX and Eomes. Furthermore, immunosuppressive cytokines such as TGF-β in the tumor microenvironment or during chronic infection further impair CD8+ T cell function. Therapeutic targeting of these inhibitory pathways offers a promising strategy to reinvigorate antitumor and antiviral immunity[11,12].
Applications and Cutting-Edge Research: Emerging strategies to harness CD8+ T cell cytotoxicity are revolutionizing the treatment of intractable diseases. In oncology, chimeric antigen receptor (CAR) T-cell therapy and T-cell receptor (TCR)-engineered T cells are being developed to redirect and potentiate CD8+ T cell specificity and killing toward defined tumor antigens. Meanwhile, in chronic viral infections like hepatitis B, recent studies show inhibiting immunosuppressive pathways (e.g., TGF-β or cAMP-PKA axis) reverses T cell dysfunction and restores potent antiviral immunity[11,12].
CD8+ T cell-mediated cytotoxicity achieves precise immune clearance through perforin-granzyme, Fas-FasL, and cytokine-dependent pathways. This mechanism is not only essential for combating pathogen infections but also indispensable for antitumor immunity and preventing chronic virus-related complications. However, regulating this process requires balancing immune clearance promotion and immune dysregulation prevention. Future research directions include modulation of CD8+ T cell activity, integrated intervention of immune checkpoints, and repair strategies for exhausted T cells, which will serve as key focuses for further optimizing immunotherapies.
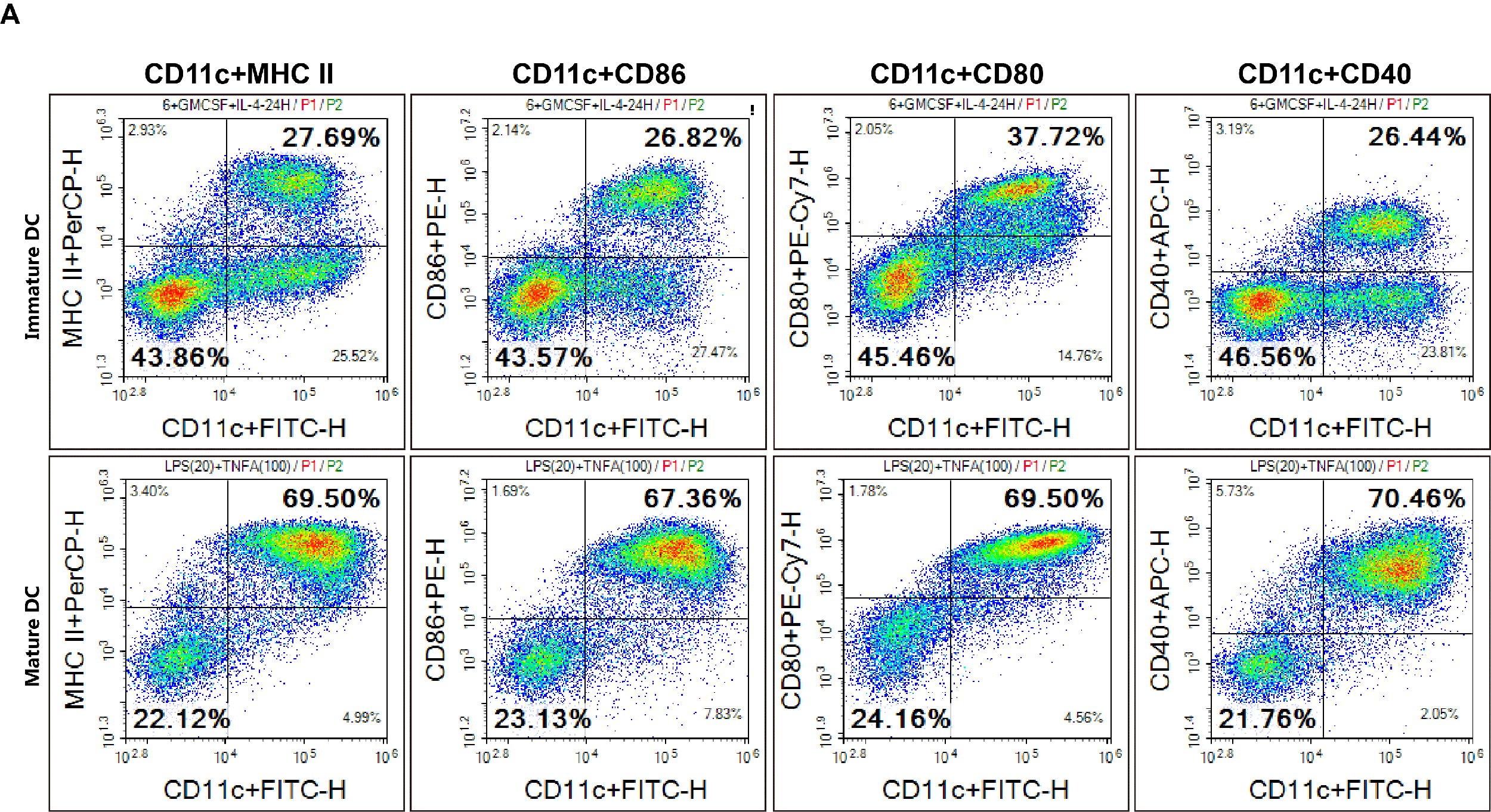
Fig. 3 The culture of matured BMDC cells: C57BL/6 splenocytes were cultured and stained with Mouse Bone Marrow-derived Dendritic Cells (BMDC) Induction and Identification Kit (XJM003), followed by analysis via flow cytometry.
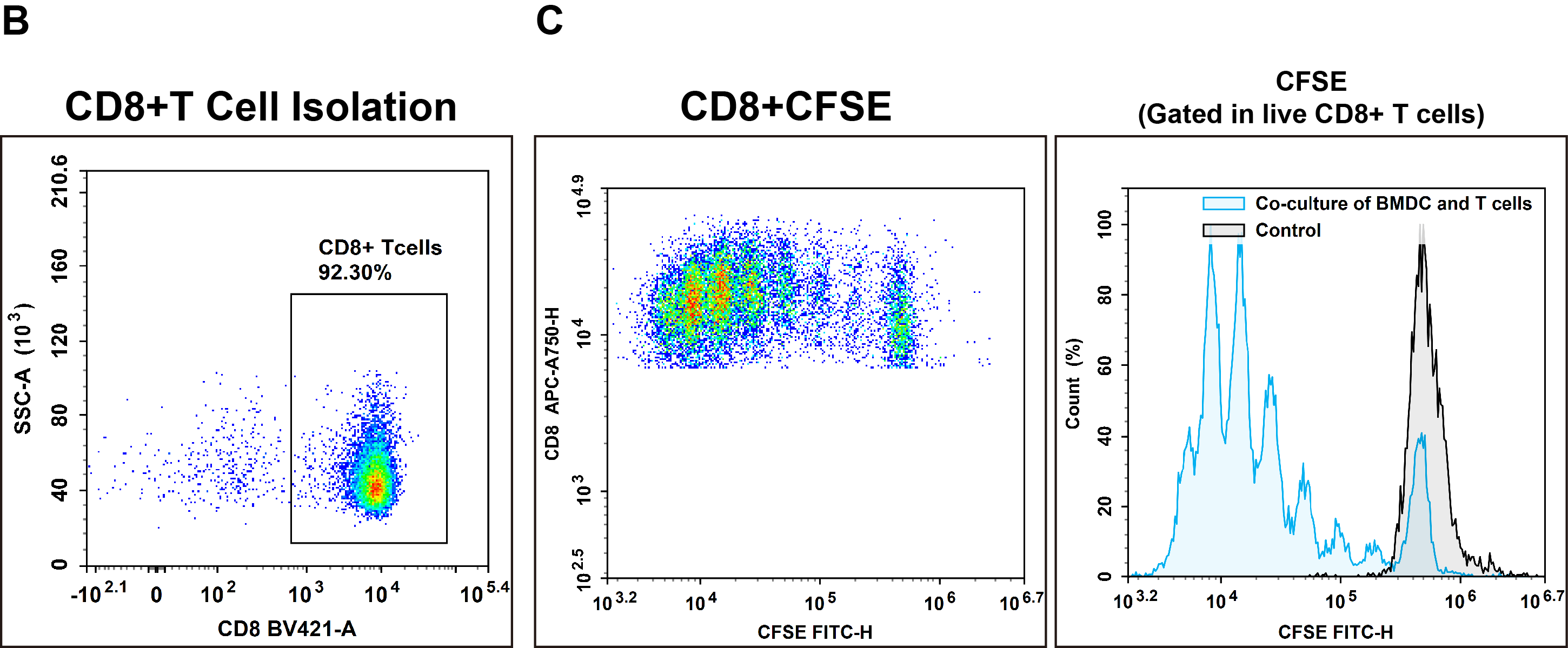
Fig. 4 Sorting, activation and proliferation of CD8 T cells: (B) CD8+ T cells were isolated from C57BL/6 splenocytes by negative selection using a specific kit (MIM003N), and their purity was assessed by flow cytometry. (C) Subsequently, the sorted CD8+ T cells were co-cultured for 72 hours with matured dendritic cells, and T cell proliferation was measured.
Elabscience® Quick Overview of Popular Products:
Table. 4 Research Tools for Cytotoxic T Cells
|
Product Name |
Cat. No. |
|
Mouse Bone Marrow-derived Dendritic Cells (BMDC) Induction and Identification Kit |
XJM003 |
|
EasySort™ Mouse CD3+T Cell Isolation Kit |
MIM001N |
|
Caspase 3/7 Activity Detection Substrate for Flow Cytometry |
E-CK-A483 |
|
CFSE Cell Division Tracker Kit |
E-CK-A345 |
06 Cytotoxic T cells and cancer immunotherapy
Cytotoxic T cells (CTLs) are closely associated with cancer immunotherapy. Their antitumor effects and applications in various therapeutic approaches have emerged as core areas in immunological research and cancer treatment. The following comprehensively analyzes this topic from two aspects: immunotherapeutic strategies and latest research progress.
6.1 Applications in Cancer Immunotherapy
Immune checkpoint inhibitors play a crucial role in reversing tumor-induced immune suppression: tumor cells evade immune surveillance via overexpressing immunosuppressive molecules (e.g., PD-L1) that interact with PD-1 on CD8+ T cells, while immune checkpoint inhibitors (e.g., anti-PD-1/PD-L1 antibodies) block this interaction to restore T cell activity and robustly enhance antitumor efficacy. Notably, anti-PD-1 antibodies such as Nivolumab and Pembrolizumab have been widely applied in the treatment of diverse malignancies, including melanoma and non-small cell lung cancer[17,18].
Chimeric antigen receptor (CAR)-T cell therapy represents a transformative approach in cancer immunotherapy: genetically engineered to express specific chimeric antigen receptors, CAR-T cells exhibit enhanced specific recognition and cytotoxicity toward target cells. While this therapy has achieved groundbreaking progress in treating hematological malignancies (e.g., B-cell acute lymphoblastic leukemia), its efficacy in solid tumor treatment remains modest, primarily attributed to the suppressive tumor microenvironment and the heterogeneous expression of tumor antigens[17,18].
Cancer Vaccines: Cancer vaccines represent a pivotal immunotherapeutic strategy, which exert preventive or therapeutic effects by eliciting CD8+ T cell-mediated immune responses against tumor-specific antigens. For instance, vaccines tailored to target co-expressed tumor antigens not only activate effector T cells but also promote the generation of long-lived memory T cells, thereby reinforcing durable antitumor immunity[19,20].
6.2 Latest Research Progress and Future Directions
Tumor Microenvironment (TME) Regulation: Tumor microenvironment (TME) regulation is critical for modulating CD8+ T cell-mediated antitumor immunity. Research findings highlight that immunosuppressive factors within the TME, such as TGF-β, exert negative regulatory effects on CD8+ T cell activity; notably, specific blockade of these signaling pathways (e.g., via TGF-β inhibitors) can substantially restore the cytotoxic functions of CD8+ T cells. Accordingly, optimizing therapeutic regimens through the combination of immune checkpoint inhibitors and TME-modulating agents remains a core focus of current research in this field[17,18].
T Cell Exhaustion and Reactivation: In the setting of chronic pathogen infection or persistent tumor antigen stimulation, CD8+ T cells often undergo exhaustion, a distinct functional state characterized by altered intracellular metabolism, diminished effector function, and impaired memory formation. Emerging therapeutic strategies aim to reactivate exhausted CD8+ T cells through targeted modulation of key transcription factors, including TOX and Eomesodermin (Eomes), which are critical regulators of the exhaustion program[17].
Genetic Engineering Optimization: Enhanced genetic modification of CD8+ T cells using state-of-the-art gene-editing technologies (e.g., CRISPR/Cas9) offers promising avenues to augment their antitumor potency. Such modifications encompass the engineering of more stable signal-amplifying receptors, targeted optimization of cellular metabolic pathways, and integration of intrinsic anti-exhaustion mechanisms to sustain long-term functional integrity[18,19].
CD8+ T cells serve as the central pillar of cancer immunotherapy, executing precise cytotoxic mechanisms to eliminate tumor cells. Their antitumor efficacy has been further amplified by innovative therapeutic strategies, including immune checkpoint inhibitors and chimeric antigen receptor (CAR)-T cell therapy. Nevertheless, overcoming technical hurdles in solid tumor treatment, resolving CD8+ T cell exhaustion, and mitigating the immunosuppressive landscape of the TME remain paramount challenges and key directions for future translational research.
References:
[1] Raskov H , Orhan A , Christensen J P ,et al.Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy[J].British Journal of Cancer, 2020.DOI:10.1038/s41416-020-01048-4.
[2] Scholer, A., Hugues, S., Boissonnas, A., Fetler, L., & Amigorena, S. (2008). Intercellular Adhesion Molecule-1-Dependent Stable Interactions between T Cells and Dendritic Cells Determine CD8+ T Cell Memory. Immunity, 28(2), 258–270. https://doi.org/10.1016/j.immuni.2007.12.016.
[3] Wang, X., & Dong, C. (2013). The CD70-CD27 Axis, a New Brake in the T Helper 17 Cell Response. Immunity, 38(1), 1–3. https://doi.org/10.1016/j.immuni.2013.01.005.
[4] Kaech, S. M., & Ahmed, R. (2001). Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nature Immunology, 2(5), 415–422. https://doi.org/10.1038/87720.
[5] Murali-Krishna, K., & Ahmed, R. (2000). Cutting Edge: Naive T Cells Masquerading as Memory Cells. The Journal of Immunology, 165(4), 1733–1737. https://doi.org/10.4049/jimmunol.165.4.1733.
[6] Quon, S. J., Yu, B., He, Z., Russ, B., Turner, S. J., Murre, C., & Goldrath, A. W. (2019). CTCF is necessary for CD8+ effector T cell differentiation. The Journal of Immunology, 202(1_Supplement), 125.6-125.6. https://doi.org/10.4049/jimmunol.202.supp.125.6.
[7] Anoveros-Barrera A , Bhullar A S , Stretch C ,et al.Immunohistochemical phenotyping of T cells, granulocytes, and phagocytes in the muscle of cancer patients: association with radiologically defined muscle mass and gene expression[J].Skeletal Muscle, 2019, 9(1).DOI:10.1186/s13395-019-0209-y.
[8] Mousset, C. M., Hobo, W., Woestenenk, R., Preijers, F., Dolstra, H., & van der Waart, A. B. (2019). Comprehensive Phenotyping of T Cells Using Flow Cytometry. Cytometry. Part A : the journal of the International Society for Analytical Cytology, 95(6), 647–654. https://doi.org/10.1002/cyto.a.23724.
[9] Wherry E J , Ha S J , Kaech S M ,et al.Molecular signature of CD8+ T cell exhaustion during chronic viral infection.[J].Immunity, 2007, 27(4):670-684.DOI:10.1016/j.immuni.2007.09.006.
[10] Mclaughlin B E , Baumgarth N , Bigos M ,et al.Nine-color flow cytometry for accurate measurement of T cell subsets and cytokine responses. Part I: Panel design by an empiric approach[J].Cytometry Part A, 2010, 73A(5):400-410.DOI:10.1002/cyto.a.20555.
[11] Zhou, J., Kryczek, I., Li, S., Li, X., Aguilar, A., Wei, S., Grove, S., Vatan, L., Yu, J., Yan, Y., Liao, P., Lin, H., Li, J., Li, G., Du, W., Wang, W., Lang, X., Wang, W., Wang, S., & Zou, W. (2021). The ubiquitin ligase MDM2 sustains STAT5 stability to control T cell-mediated antitumor immunity. Nature Immunology, 22(4), 460–470. https://doi.org/10.1038/s41590-021-00888-3.
[12] Fumagalli, V., & Iannacone, M. (2024). Unlocking CD8+ T cell potential in chronic hepatitis B virus infection. Nature Reviews Gastroenterology & Hepatology, 22(2), 92–93. https://doi.org/10.1038/s41575-024-01015-x.
[13] Abusamra A J , Zhong Z , Zheng X ,et al.Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis.[J].Annals of Surgical Oncology, 2005, 35(2):169-173.DOI:10.1016/j.bcmd.2005.07.001.
[14] Yasukawa M , Ohminami H , Arai J ,et al.Granule exocytosis, and not the fas/fas ligand system, is the main pathway of cytotoxicity mediated by alloantigen-specific CD4(+) as well as CD8(+) cytotoxic T lymphocytes in humans.[J].Blood, 2000, 95(7):2352-2355.DOI:10.1007/s002770050583.
[15] Slifka M K , Rodriguez F , Whitton J L .Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells[J].Nature, 1999, 401(6748):76-79.DOI:10.1038/43454.
[16] Michael,A,Derby,et al.An abrupt and concordant initiation of apoptosis: antigen-dependent death of CD8+ CTL[J].European Journal of Immunology, 2001.DOI:10.1002/1521-4141(2001010)31:10<2951::AID-IMMU2951>3.0.CO.
[17] He, L., Chen, N., Dai, L., & Peng, X. (2023). Advances and challenges of immunotherapies in NK/T cell lymphomas. iScience, 26(11), 108192. https://doi.org/10.1016/j.isci.2023.108192.
[18] Wang, H., Song, X., Shen, L., Wang, X., & Xu, C. (2022). Exploiting T cell signaling to optimize engineered T cell therapies. Trends in Cancer, 8(2), 123–134. https://doi.org/10.1016/j.trecan.2021.10.007.
[19] Jou, E. (2024). Clinical and basic science aspects of innate lymphoid cells as novel immunotherapeutic targets in cancer treatment. In Progress in Molecular Biology and Translational Science (pp. 1–60). Elsevier. https://doi.org/10.1016/bs.pmbts.2024.03.036.
[20] Gauthier, L., & Vivier, E. (2020). Boosting Cytotoxic Antibodies against Cancer. Cell, 180(5), 822–824. https://doi.org/10.1016/j.cell.2020.02.025.
[21] Giles J R , A.-M. G , Kaech S M W E J .CD8~+ T cells in the cancer-immunity cycle[J].Immunity, 2023, 56(10):2231-2253.DOI:10.1016/j.immuni.2023.09.005.



