In flow cytometry experiments, we not only need to focus on the rational pairing of fluorescent antibodies but also select appropriate related reagents to achieve optimal staining. In multicolor flow cytometry, the proper use of related reagents can reduce fluorescence background, enhance signal specificity, and directly impact the accuracy and reproducibility of experimental results. In this guide, we will explain how to utilize flow cytometry related reagents to achieve better experimental outcomes.
Table of Contents
1. Sample Processing Reagents
2. Staining Reagents
3. Cell Viability Reagents
Fig. 1 Flow Cytometry Auxiliary Reagents and Respective Functions.
01 Sample Processing Reagents
1.1 Sample Processing
The primary goal of sample processing is to pre-treat samples to meet the requirements for flow cytometric analysis. This involves obtaining a single-cell suspension while ensuring sufficient target cell numbers, optimal cell viability, and intact antigen epitopes. During sample processing, red blood cell lysis buffer can be used to eliminate red blood cells and prevent interference with experimental results. Alternatively, Ficoll separation medium may be employed to isolate target cells.
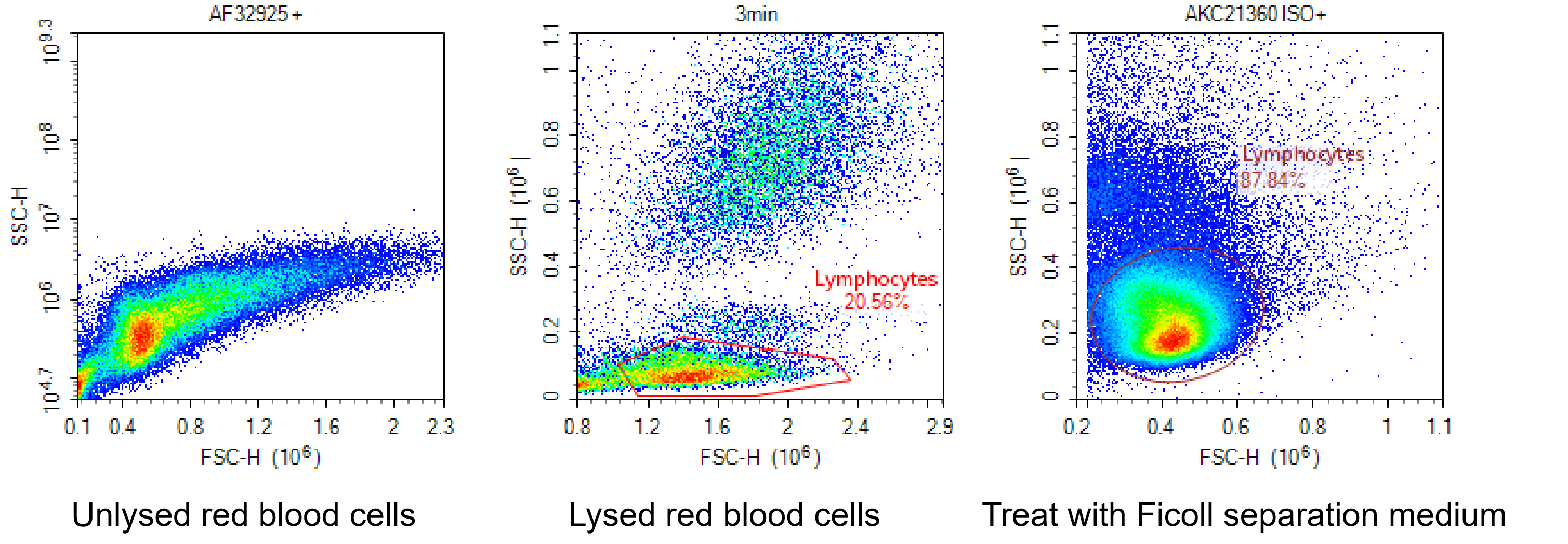
Fig. 2 Detection Results of Lymphocytes under Different Treatments (Left: Untreated with Red Blood Cell Lysis; Middle: Treated with Red Blood Cell Lysis Buffer; Right: Treated with Ficoll Separation Medium).
1.2 T Cells Activation and Expansion
To evaluate the function and state of T cells, activation and proliferation are typically required prior to detection. Anti-CD3 and anti-CD28 antibodies serve as the primary and secondary signals for T cell activation. Using magnetic beads conjugated with CD3/CD28 mimics the natural activation mechanism of antigen-presenting cells (APCs). Compared to the traditional method of directly adding antibodies to the culture medium, this approach more closely resembles the physiological activation process, providing sustained and stable stimulation signals while reducing the risk of T cell exhaustion.
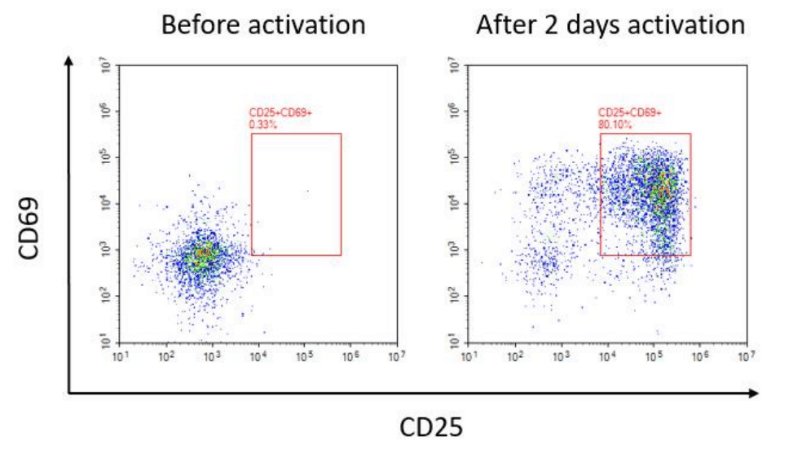
Fig. 3 Expression Results of CD25 and CD69 in Human T Cells Before Activation and After 2 Days of Activation.
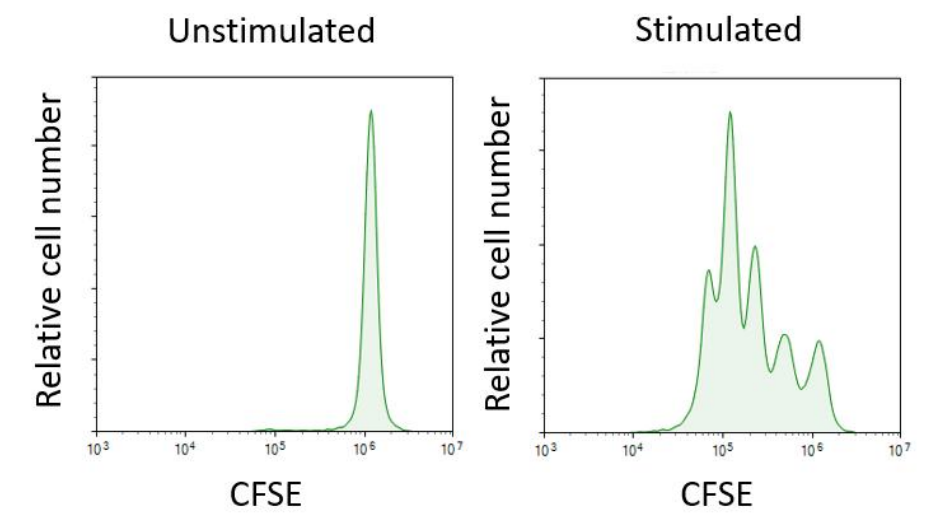
Fig. 4 Proliferation Results of T Cells in Non-activated and Activated Groups after 3 Days of Culture (with IL-2 Cytokine Supplementation), Following CFSE Staining.
02 Staining Reagents
2.1 Staining Buffer
Flow cytometry staining buffers contain BSA, which helps reduce nonspecific binding of antibodies or fluorescent dyes to cells. They also mitigate shearing forces between cells during procedures such as centrifugation, thereby protecting cells and minimizing cell loss. This makes them particularly suitable for intracellular flow cytometry staining.
2.2 Fc Receptor Blocking Reagents
Using Fc receptor blockers can reduce nonspecific antibody binding and optimize the signal-to-noise ratio.
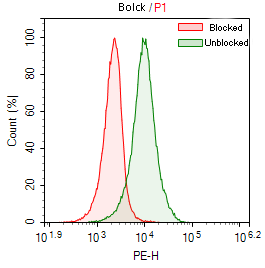
Fig. 5 Results of Fc Receptor Blocking Treatment. Green: THP-1 cells stained with PE Mouse IgG2a, κ Isotype Control [C1.18.4] without using an Fc receptor blocking reagent. Red: THP-1 cells stained with PE Mouse IgG2a, κ Isotype Control [C1.18.4] after using EasyStain™ Human Fc Receptor Blocking Solution.
2.3 Intracellular Cytokine Staining
Intracellular cytokine protein detection requires specific stimulators, inhibitors, and fixation/permeabilization reagents. Stimulators activate T cells and promote cytokine secretion, while inhibitors block cytokines within the Golgi apparatus, preventing their release extracellularly. Fixation and permeabilization reagents not only effectively facilitate the detection of intracellular signals but also minimize damage to surface antigen markers.
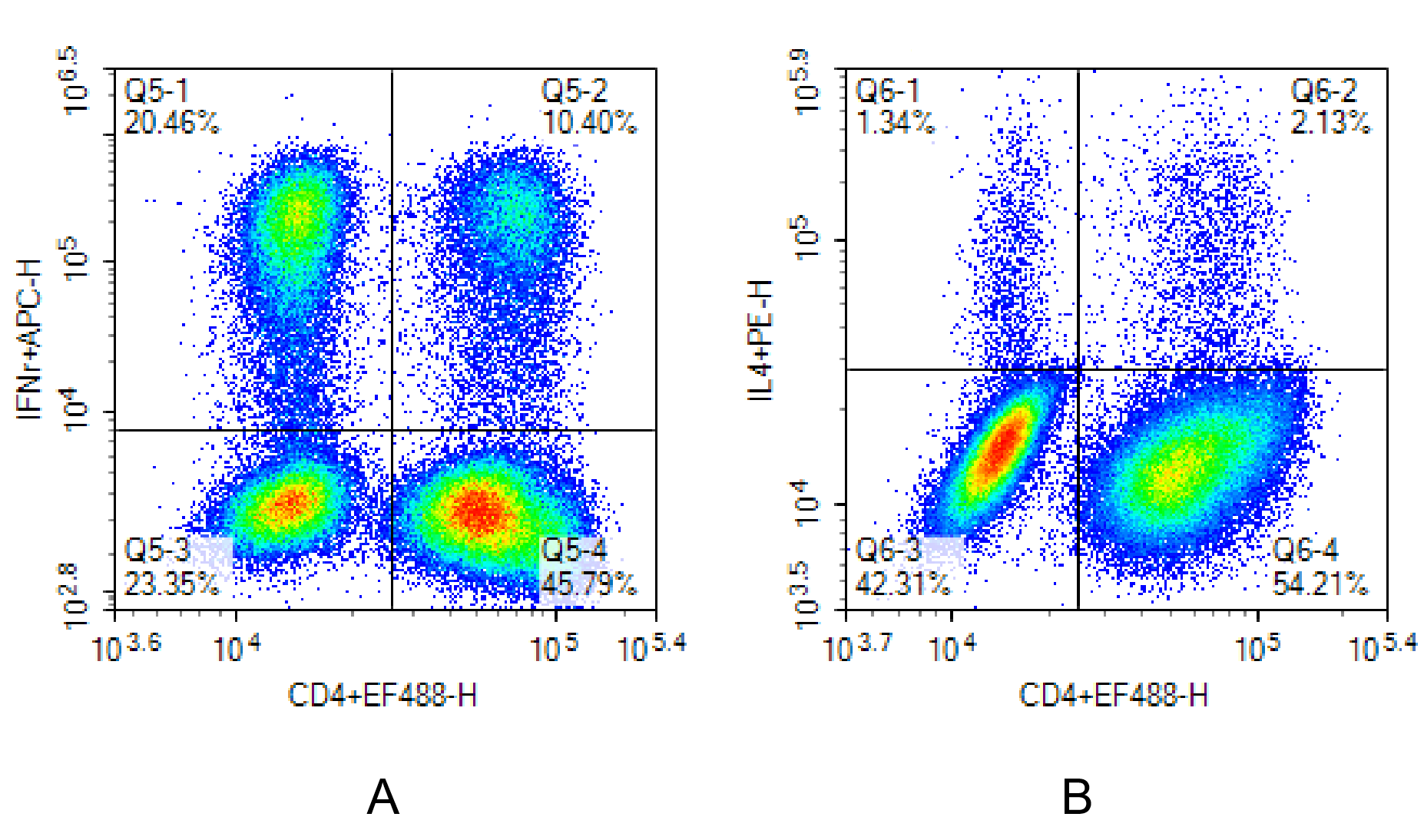
Fig. 6 Human PBMCs were stimulated with the Cell Stimulation and Protein Transport Inhibitor Kit for 6 hours, then stained with Elab Fluor® 488 Anti-Human CD4 Antibody [SK3]. After fixation and permeabilization using the Intracellular Fixation/Permeabilization Buffer Kit, intracellular staining was performed with APC Anti-Human IFN-γ Antibody [B27] (A) and PE Anti-Human IL-4 Antibody [MP4-25D2] (B).
2.4 Intranuclear Cytokine Staining
Intranuclear factor staining requires the use of fixation and nuclear membrane permeabilization reagents. Nuclear markers such as Foxp3, RORγt, and Bcl-6 can be detected simultaneously and are compatible with cytokine markers like IFN-γ, IL-4, and IL-17A.
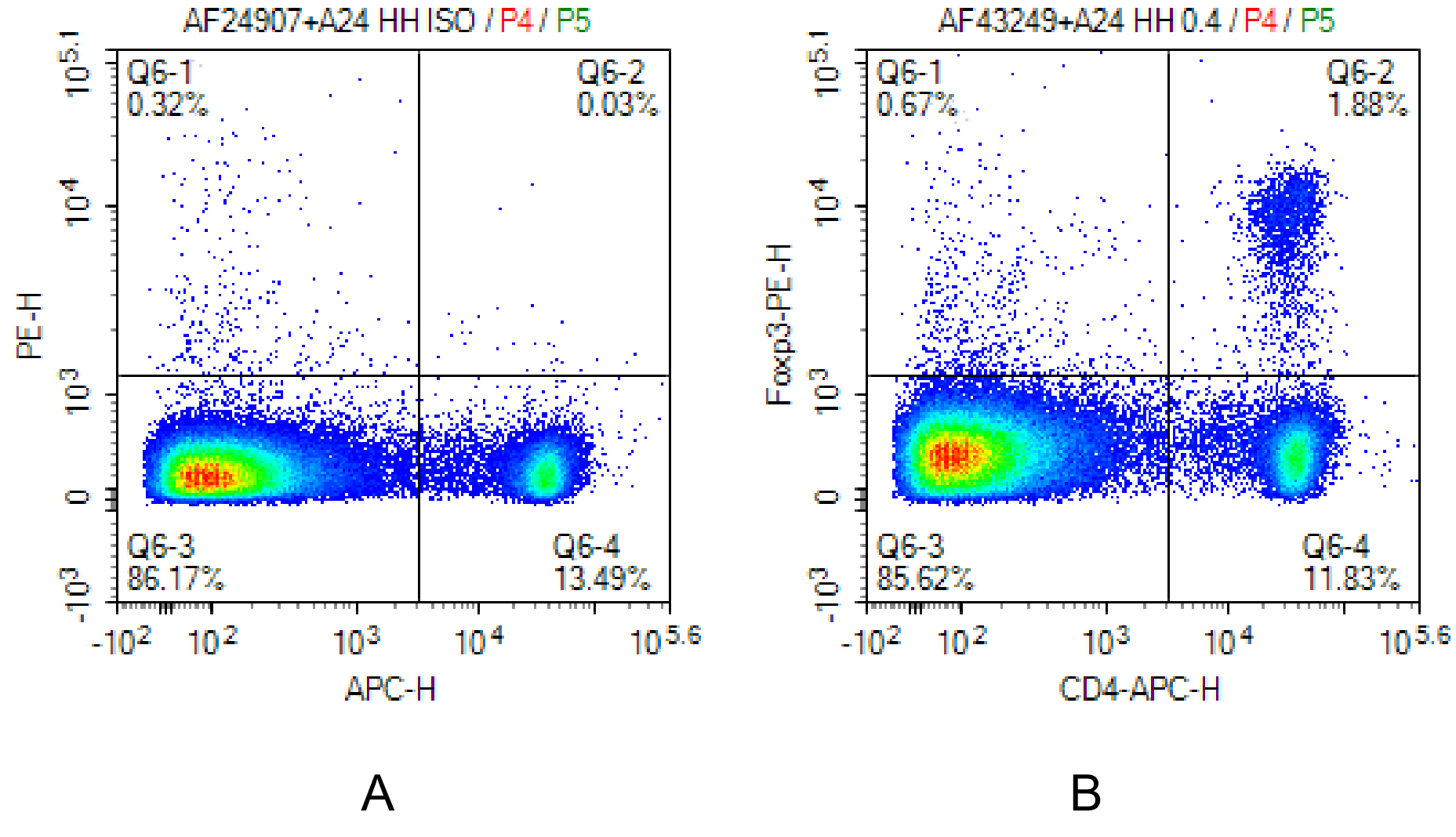
Fig. 7 Mouse spleen cells were surface-stained with APC Anti-Mouse CD4 Antibody[GK1.5]. Following fixation and permeabilization using the Foxp3/Transcription Factor Staining Kit, intracellular staining was performed with PE Anti-Mouse Foxp3 Antibody[3G3] (B) and PE Mouse IgG1, κ Isotype Control[MOPC-21] (A).
03 Cell Viability Reagents
Viability dyes are primarily categorized into nuclear dyes and amine-reactive dyes. Nuclear dyes utilize the selective permeability of live cell membranes to distinguish dead cells from live ones, making them suitable only for surface staining flow cytometry experiments and incompatible with intracellular or intranuclear flow cytometry assays. For samples requiring fixation and permeabilization, protein-reactive dyes, such as amine-reactive dyes, are necessary. Amine-reactive dyes bind to primary amines on the surface of live cells. When cell death occurs and membrane integrity is compromised, these dyes can bind to both surface and intracellular primary amines, resulting in higher fluorescence intensity in dead cells compared to live cells, thereby enabling the distinction between live and dead cells.
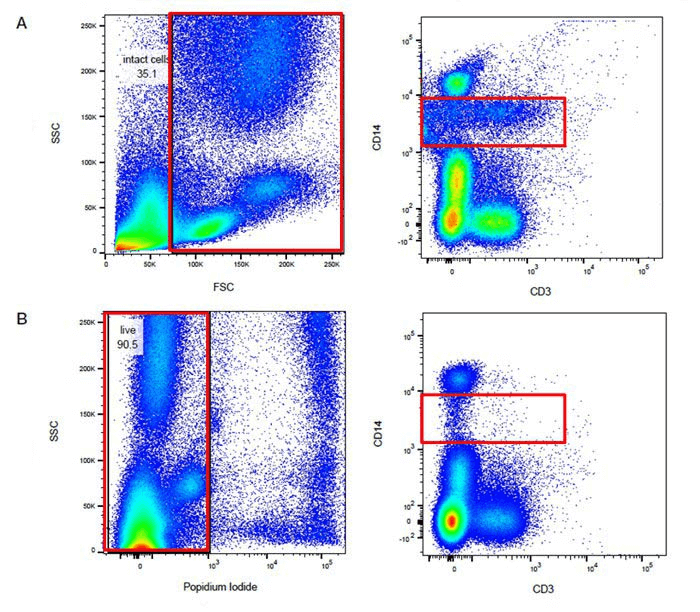
Fig. 8 Comparison of Results from the Same Sample before and after Dead Cell Exclusion (Group A: Without Viability Dye Staining; Group B: Stained with PI Dye). Dead cells are prone to nonspecific binding with antibodies or fluorochromes, which can affect the proportion of target cells.
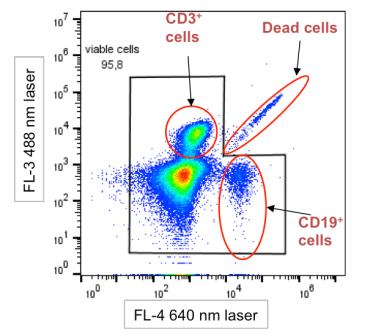
Fig. 9 Schematic Diagram of Dead Cell Interference Characteristics. Dead cells distribute along the diagonal on the plot (Dead cells). The autofluorescence of dead cells generates signals across multiple fluorescence channels, with significant interference observed in short-wavelength (blue/green) channels. This often manifests as a "comet tail" pattern or diffuse trailing and spreading toward higher fluorescence intensities.
Table 1. Elabscience Flow Cytometry Antibody Related Reagent Product List
|
Product Category |
Product Name |
Cat. No. |
Size |
|
Red Blood Cell Lysis |
10×ACK Lysis Buffer |
E-CK-A105 |
100 mL/200 mL /500 mL |
|
10× RBC Lysis/Fixation Solution |
E-CK-A106 |
50 Tests/100 Tests / 500 Tests |
|
|
Ficoll |
Human PBMC Separation Solution(P 1.077) |
E-CK-A103 |
200 mL |
|
T Cell Activation and Expansion |
Human CD3/CD28 T Cell Activation Beads |
MIH001A |
0.2 mL /1 mL / 1 mL×5 |
|
Mouse CD3/CD28 T Cell Activation Beads |
MIM001A |
0.2 mL /1 mL / 1 mL×5 |
|
|
Buffer |
Cell Staining Buffer |
E-CK-A107 |
100 mL/200 mL /500 mL |
|
FcR Blocking |
Purified Anti-Mouse CD16/32 Antibody[2.4G2] |
E-AB-F0997A |
25 μg/100 μg |
|
EasyStainTM Human Fc Receptor Blocking Solution |
E-CK-A171 |
50 Tests/200 Tests |
|
|
Cell Stimulation and Protein Transport Inhibitor |
Cell Stimulation and Protein Transport Inhibitor Kit |
E-CK-A091 |
50 Assays/100 Assays /200 Assays /500 Assays |
|
Cell Stimulation MIX Kit |
E-CK-A019 |
50 Assays/200 Assays /500 Assays |
|
|
Protein Transport Inhibitor MIX |
E-CK-A013 |
50 Tests/200 Tests / 500 Tests |
|
|
Cell Fixation/ Permeabilization |
Intracellular Fixation/Permeabilization Buffer Kit |
E-CK-A109 |
50 Assays/100 Assays/ 500 Assays |
|
Foxp3/Transcription Factor Staining Kit |
E-CK-A108 |
20 Assays |
|
|
Nuclear Staining |
PI Reagent (50μg/mL) |
E-CK-A161 |
50 Tests/100 Tests / 200 Tests/500 Tests |
|
7-AAD Reagent (100μg/mL) |
E-CK-A162 |
50 Tests/100 Tests / 200 Tests/500 Tests |
|
|
DAPI Reagent (25μg/mL) |
E-CK-A163 |
50 Tests/100 Tests / 200 Tests/500 Tests |
|
|
Cell Viability Dye |
STYX™ Green Fixable Viability Kit |
E-CK-A166 |
50 Tests/100 Tests / 200 Tests |
|
STYX™ Violet Fixable Viability Kit |
E-CK-A167 |
50 Tests/100 Tests / 200 Tests |



