Background
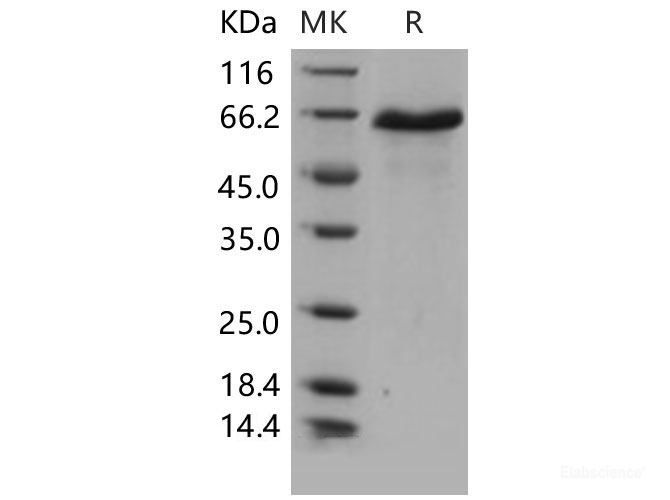
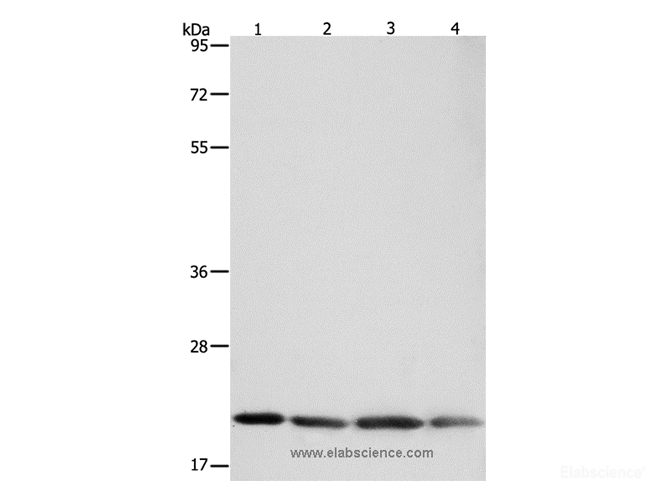
Delta-like protein 1(DLL1), also known as Delta1, a single-pass type I membrane protein which contains one DSL domain and eight EGF-like domains, acts as a ligand for Notch receptors, and positively regulates T-cell development. DLL1 is proteolytically processed in a similar manner to the Notch receptor, and it has been speculated to participate in bidirectional signaling. The proteolytic processing of DLL1 helps achieve an asymmetry in Notch signaling in initially equivalent myogenic cells and helps sustain the balance between differentiation and self-renewal. Interactions between DLL1 and Notch in trans activate the Notch pathway, whereas DLL1 binding to Notch in cis inhibits Notch signaling. DLL1 undergoes proteolytic processing in its extracellular domain by ADAM10. It had been demonstrated that DLL1 represents a substrate for several other members of the ADAM family. In co-transfected cells, DLL1 is constitutively cleaved by ADAM12, and the N-terminal fragment of DLL1 is released to medium. ADAM12-mediated cleavage of DLL1 is cell density-dependent, takes place in cis orientation, and does not require the presence of the cytoplasmic domain of ADAM12. Full-length DLL1, but not its N- or C-terminal proteolytic fragment, co-immunoprecipitates with ADAM12. By using a Notch reporter construct, we show that DLL1 processing by ADAM12 increases Notch signaling in a cell-autonomous manner. Furthermore, ADAM9 and ADAM17 have the ability to process DLL1. In contrast, ADAM15 does not cleave DLL1, although the two proteins still co-immunoprecipitate with each other.